Abstract
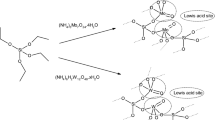
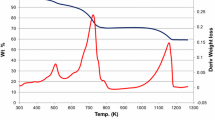
Transesterification of diethyl oxalate (DEO) with phenol over MoO3-SiO2 catalysts prepared by the sol-gel technique (MoO3-SiO2 (SG)) and the impregnation method (MoO3-SiO2 (I)) was conducted to produce diphenyl oxalate (DPO), which can be used as a precursor for manufacturing diphenyl carbonate (DPC). The sample MoO3-SiO2 (SG) containing 12 wt % of MoO3 showed the best performance with 71.0% conversion of DEO and 32.0% selectivity to DPO. Compared to MoO3-SiO2 (I), improvements in the DEO conversion and DPO selectivity with MoO3-SiO2 (SG) were 16.1 and 7%, respectively. Crystal structure and phase composition of MoO3-SiO2 (I) and MoO3-SiO2 (SG) catalysts with varying MoO3 contents were investigated. The sample MoO3-SiO2 (SG) with a similar chemical composition to MoO3-SiO2 (I) has a larger specific surface area, indicating that the active component is well dispersed on the surface of the MoO3-SiO2 (SG) catalysts. Results of XRD and XPS measurements suggest a high degree of dispersion of MoO3-SiO2 (SG) catalysts that can account for an increase in DEO conversion and DPO selectivity. Coordinately unsaturated MoO3 species play a significant role in the catalytic performance of MoO3-SiO2 (SG) catalysts in transesterification of DEO with phenol. In addition, IR measurements of pyridine adsorption and NH3-TPD data indicate that the amount of acid sites on the surface of MoO3-SiO2 (SG) exceeds that found for the surface of MoO3-SiO2 (I). An enhanced concentration of surface MoO3 species in tetrahedral coordination coupled with the presence of weak Lewis acid sites appear to be the main reason why MoO3-SiO2 (SG) catalysts are superior to the MoO3-SiO2 (I) system.
Similar content being viewed by others
References
Freitag, D., Grico, U., Muller, P.R., and Nouvertne, W., in Encyclopedia of Polymer Science and Engineering, Mark, H.F., Ed., New York Wiley, 1987, vol. 11, p. 649.
Sikdar, S.K., Chem. Tech., 1987, vol. 17, p. 112.
Janatpour, M. and Shafer, S.J., Eur. Patent 228672, 1987.
Ishii, H., Goyal, M., and Takeuchi, K., J. Mol. Catal. A: Chem., 1999, vol. 144, p. 477.
Shaikh, A.G. and Sivaram, S., Chem. Rev., 1996, vol. 96, p. 951.
Fu, Z.H. and Ono, Y., J. Mol. Catal. A: Chem., 1997, vol. 118, p. 293.
Kim, W.B. and Lee, J.S., Catal. Lett., 1999, vol. 59, p. 83.
Kim, W.B., Kim, Y.G., and Lee, S., Appl. Catal., A, 2000, vols. 194–195, p. 403.
Ono, Y., Appl. Catal., A, 1997, vol. 155, p. 133.
Wang, S.P., Liu, Y., Shi, Y., Ma, X.B., and Gong, J.L., AIChE J., 2008, vol. 54, p. 741.
Liu, Y., Ma, X.B., Wang, S.P., and Gong, J.L., Appl. Catal., B, 2007, vol. 77, p. 125.
Keigo, N., Shuji, T., Katsumasa, H., and Ryoji, S., US Patent 5834651, 1998.
Keigo, N., Shuji, T., Katsumasa, H., Ryoji, S., Akinori, S., and Katsutoshi, W., US Patent 5922827, 1999.
Ma, X.B., Gong, J.L., Wang, S.P., Gao, N., Wang, D.L., and Yang, X., Catal. Commun., 2004, vol. 5, p. 101.
Gong, J.L., Ma, X.B., Yang, X., Wang, S.P., and Wen, S.D., Catal. Commun., 2004, vol. 5, p. 179.
Wang, S.P., Ma, X.B., Gong, J.L., Yang, X., Guo, H.L., and Xu, G.H., Ind. Eng. Chem. Res., 2004, vol. 43, p. 4027.
Ma, X.B., Guo, H.L., Wang, S.P., and Sun, Y.L., Fuel Process. Technol., 2003, vol. 83, p. 275.
Gong, J.L., Ma, X.B., Wang, S.P., Liu, M.Y., Yang, X., and Xu, G.H., J. Mol. Catal. A: Chem., 2004, vol. 207, p. 215.
Wang, S.P., Ma, X.B., Guo, H.L., Gong, J.L., Yang, X., and Xu, G.H., J. Mol. Catal. A: Chem., 2004, vol. 214, p. 273.
Biradar, A.V., Umbarkar, S.B., and Dongare, M.K., Appl. Catal., A, 2005, vol. 285, p. 190.
Bian, L., Wang, S.P., and Ma, X.B., J. Chin. Pet. Technol., 2009, vol. 38, p. 754.
Williams, C.C., Ekerdt, J.G., Jehng, J.M., Hardcastle, F.D., and Wach, I.E., J. Phys. Chem., 1991, vol. 95, p. 8781.
Carbucicchio, M. and Triro, F., J. Catal., 1980, vol. 62, p. 13.
Bruckman, K., Grzybowska, B., Che, M., and Tatibouet, J.M., Appl. Catal., A, 1993, vol. 96, p. 279.
Ono, T., Miyata, H., and Kubokawa, Y., J. Chem. Soc., Faraday Trans., 1987, vol. 83, p. 1761.
Zhao, B.Y., Xu, Q., Gui, L.L., and Tang, Y.Q., Acta Chim. Sinica, 1990, vol. 48, p. 227.
Knözinger, H., Science, 2000, vol. 287, p. 1407.
Knözinger, H., Adv. Catal., 1976, vol. 25, p. 184.
Reddy, B.M., Chowdhury, B., and Smirniotis, P.G., Appl. Catal., A, 2001, vol. 211, p. 19.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Bian, L., Wang, S.P. & Ma, X.B. The effect of catalyst preparation on the activity of MoO3-SiO2 catalyst in transesterification of diethyl oxalate. Kinet Catal 55, 763–769 (2014). https://doi.org/10.1134/S0023158414060032
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158414060032