Abstract
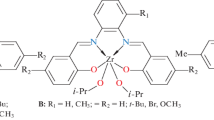
The number of active centers C p in the homogeneous complexes LCoCl2 and LVCl3 (L = 2,6-(2,6-R2C6H3N=CMe)2C5H3N; R = Me, Et, tBu) and the propagation rate constants k p have been determined by the radioactive 14CO quenching of ethylene polymerization on these complexes in the presence of the methylaluminoxane (MAO) activator. For the systems studied, a significant portion of the initial complex (up to 70%) transforms into polymerization-active centers. The catalysts based on the cobalt complexes are single-site, and the constant k p in these systems is independent of the volume of substituent R in the ligand, being (2.4−3.5) × 103 L mol−1 s−1 at 35°C. The much larger molecular weight of the polymer formed on the complex with the tert-butyl substituent in the aryl rings of the ligand compared to the product formed on the complex with the methyl substituent is due to the substantial (∼11-fold) decrease in the rate constant of chain transfer to the monomer. At the early stages of the reaction (before 5 min), the vanadium complexes contain active centers of one type only, for which k p = 2.6 × 103 L mol−1 s−1 at 35°C. An increase in the polymerization time to 20 min results in the appearance, in the vanadium systems, of new, substantially less reactive centers on which high-molecular-weight polyethylene forms. The number of active centers C p in the 2,5-tBu2LCoCl2 and 2,6-Et2LVCl3 systems with the MAO activator increases as the polymerization temperature is raised from 25 to 60°C. The activation energies of the chain propagation reaction (E p) have been calculated. The value of E p for complex 2,5-tBu2LCoCl2 is 4.5 kcal/mol. It is assumed that the so-called “dormant” centers form in ethylene polymerization on the 2,6-Et2LVCl3 complex, and their proportion increases with a decrease in the polymerization temperature. Probably, the anomalously high value E p = 14.2 kcal/mol for the vanadium system is explained by the formation of these “dormant” centers.
Similar content being viewed by others
References
Britovsek, G.J., Gibson, V.C., Kimberley, B.S., Maddox, P.J., McTavish, S.J., Solan, G.A., White, A.J., and Williams, D.J., Chem. Commun., 1998, no. 7, p. 849.
Small, B.L., Brookhart, M., and Bennet, A.M., J. Am. Chem. Soc., 1998, vol. 120, no. 16, p. 4049.
Reardon, D., Conan, F., Gambarotta, S., Yap, G., and Wang, Q.Y., J. Am. Chem. Soc., 1999, vol. 121, no. 40, p. 9318.
Schmidt, R., Welch, M.B., Knudsen, R.D., Gottfried, S., and Alt, H.G., J. Mol. Catal. A: Chem., 2004, vol. 222, nos. 1–2, p. 9.
Britovsek, G.J., Bruse, M., Gibson, V.C., Kimberley, B.S., Maddox, P.J., Mastroianni, S., McTavish, S.J., Redshaw, C., Solan, G.A., Stromberg, S., White, A.J., and Williams, D.J., J. Am. Chem. Soc., 1999, vol. 121, no. 38, p. 8728.
Soshnikov, I.E., Semikolenova, N.V., Bushmelev, A.N., Bryliakov, K.P., Lyakin, O.Y., Redshaw, C., Zakharov, V.A., and Talsi, E.P., Organometallics, 2009, vol. 28, no. 20, p. 6003.
Kim, I., Han, B.H., Ha, Y.S., Ha, C.S., and Park, D.W., Catal. Today, 2004, vol. 93–95, p. 281.
Kim, I., Han, B.H., Kim, J.S., and Ha, C.S., Macromol. Res., 2005, vol. 13, no. 1, p. 2.
Liu, J.Y., Zheng, Y., Li, Y.G., Pan, L., Li, Y.S., and Hu, N.H., J. Organomet. Chem., 2005, vol. 690, no. 5, p. 1233.
Schmidt, R., Welch, M.B., Knudsen, R.D., Gottfried, S., and Alt, H.G., J. Mol. Catal. A: Chem., 2004, vol. 222, nos. 1–2, p. 17.
Semikolenova, N.V., Zakharov, V.A., Echevskaja, L.G., Matsko, M.A., Bryliakov, K.P., and Talsi, E.P., Catal. Today, 2009, vol. 144, nos. 3–4, p. 334.
Colamarco, E., Milione, S., Cuomo, C., and Grassi, A., Macromol. Rapid Commun., 2004, vol. 25, no. 2, p. 450.
Semikolenova, N.V., Zakharov, V.A., Talsi, E.P., Babushkin, D.E., Sobolev, A.P., Echevskaya, L.G., and Khusniyarov, M.M., J. Mol. Catal. A: Chem., 2002, vols. 182–183, nos. 1–2, p. 283.
Kooistra, T.M., Knijnenburg, Q., Smits, J.M.M., Horton, A.D., Budzelaar, P.H.M., and Gal, A.W., Angew. Chem. Int. Ed., 2001, vol. 40, no. 24, p. 4719.
Barabanov, A.A., Bukatov, G.D., Zakharov, V.A., Semikolenova, N.V., Echevskaja, L.G., and Matsko, M.A., Macromol. Chem. Phys., 2005, vol. 206, no. 22, p. 2292.
Barabanov, A.A., Bukatov, G.D., Zakharov, V.A., Semikolenova, N.V., Echevskaja, L.G., and Matsko, M.A., Macromol. Chem. Phys., 2008, vol. 209, no. 24, p. 2510.
Wu, J., Pan, Q., and Rempel, G.L., J. Appl. Polym. Sci., 2005, vol. 96, no. 3, p. 645.
Barabanov, A.A., Bukatov, G.D., and Zakharov, V.A., J. Polym. Sci., Part A: Polym. Chem., 2008, vol. 46, no. 19, p. 6621.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Barabanov, N.V. Semikolenova, M.A. Mats’ko, V.A. Zakharov, 2013, published in Kinetika i Kataliz, 2013, Vol. 54, No. 4, pp. 500–506.
Rights and permissions
About this article
Cite this article
Barabanov, A.A., Semikolenova, N.V., Mats’ko, M.A. et al. Kinetic regularities of catalytic ethylene polymerization on single- and multi-site cobalt and vanadium bis(imino)pyridine complexes. Kinet Catal 54, 475–480 (2013). https://doi.org/10.1134/S0023158413040022
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158413040022