Abstract
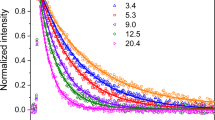
Gas-phase reactions typical of the Earth’s atmosphere have been studied for a number of partially fluorinated alcohols (PFAs). The rate constants of the reactions of CF3CH2OH, CH2FCH2OH, and CHF2CH2OH with fluorine atoms have been determined by the relative measurement method. The rate constant for CF3CH2OH has been measured in the temperature range 258–358 K (k = (3.4 ± 2.0) × 1013exp(−E/RT) cm3 mol−1 s−1, where E = −(1.5 ± 1.3) kJ/mol). The rate constants for CH2FCH2OH and CHF2CH2OH have been determined at room temperature to be (8.3 ± 2.9) × 1013 (T = 295 K) and (6.4 ± 0.6) × 1013 (T = 296 K) cm3 mol−1 s−1, respectively. The rate constants of the reactions between dioxygen and primary radicals resulting from PFA + F reactions have been determined by the relative measurement method. The reaction between O2 and the radicals of the general formula C2H2F3O (CF3CH2Ȯ and CF3ĊHOH) have been investigated in the temperature range 258–358 K to obtain k = (3.8 ± 2.0) × 108exp(−E/RT) cm3 mol−1 s−1, where E = −(10.2 ± 1.5) kJ/mol. For the reaction between O2 and the radicals of the general formula C2H4FO (Ċ HFCH2O, CH2FĊHOH, and CH2FCH2Ȯ) at T = 258–358 K, k = (1.3 ± 0.6) × 1011exp(−E/RT) cm3 mol−1 s−1, where E = −(5.3 ± 1.4) kJ/mol. The rate constant of the reaction between O2 and the radicals with the general formula C2H3F2O (ĊF2CH2O, CHF2ĊHOH, and CHF2CH2Ȯ) at T = 300 K is k = 1.32 × 1011 cm3 mol−1 s−1. For the reaction between NO and the primary radicals with the general formula C2H2F3O (CF3CH2Ȯ and CF3ĊHOH), which result from the reaction CF3CH2OH + F, the rate constant at 298 K is k = 9.7 × 109 cm3 mol−1 s−1. The experiments were carried out in a flow reactor, and the reaction mixture was analyzed mass-spectrometrically. A mechanism based on the results of our studies and on the literature data has been suggested for the atmospheric degradation of PFAs.
Similar content being viewed by others
References
Hack, W., Hold, M., Hoyermann, K., et al., Proc. EUROTRAC 98, Southampton, 1999, vol. 1, p. 99.
Papagiannakopoulos, P., Sidebottom, H., and Morozov, I., Proc. Eur. Commission, Rep. no. 67, Luxemburg, 1999, p. 99.
Belotcerkovetc, S., Hack, W., Hold, M., et al., Proc. Eur. Commission, Report no. 67, Luxemburg, 1999, p. 147.
Kelly, T., Sidebottom, H., Manning, M., et al., Proc. EUROTRAC-2 2000, Heidelberg, 2001.
Wallington, T.J., Dagout, P., and Kurilo, M.J., J. Phys. Chem., 1988, vol. 92, p. 5024.
Meier, U., Grotheer, H.H., Riekert, G., et al., Chem. Phys. Lett., 1985, vol. 115, p. 221.
Papadimitriou, V.C., Prosmitis, A.V., Lazarou, Y.G., et al., 8th Eur. Symp. on the Physico-Chemical Behaviour of Atmospheric Pollutants, Torino, 2001, p. AP126.
Manning, M., Treacy, J., and Sidebottom, H., Proc. EUROTRAC 98, Southampton, 1999, vol. 1, p. 153.
Atkinson, R., Baulch, D.L., Cox, R.A., et al., J. Phys. Chem. Ref. Data, 1997, vol. 26, p. 521.
Nottingham, W.C., Rudolph, R.N., Andrews, K.P., et al., Int. J. Chem. Kinet., 1994, vol. 26, p. 749.
Troe, J., Ber. Bunsen-Ges. Phys. Chem., 1983, vol. 87, p. 161.
Fenter, F.F., Lightfoot, P.D., Caralp, F., et al., J. Phys. Chem., 1993, vol. 97, p. 4695.
Nacke, F., Dissertation, Göttingen: Göttingen Univ., 1998.
Beiderhase, Th., Dissertation, Göttingen Univ., 1995.
Dobe, S., Lendvay, G., Szilagyi, I., et al., Int. J. Chem. Kinet., 1994, vol. 26, p. 887.
Miyoshi, A., Matsui, H., and Washida, N., Chem. Phys. Lett., 1989, vol. 160, p. 291.
Ley, L., Masanet, J., Caralp, F., et al., J. Phys. Chem., 1995, vol. 99, p. 1953.
Scientific Assessment of Ozone Depletion: 1994, World Meteorological Organization, Global Ozone Research and Monitoring Project, Report no. 37, Geneva, 1995.
Author information
Authors and Affiliations
Additional information
Original Russian Text © E.S. Vasil’ev, I.I. Morozov, W. Hack, K.-H. Hoyermann, M. Hold, 2006, published in Kinetika i Kataliz, 2006, Vol. 47, No. 6, pp. 859–870.
Rights and permissions
About this article
Cite this article
Vasil’ev, E.S., Morozov, I.I., Hack, W. et al. Kinetics and mechanism of atmospheric reactions of partially fluorinated alcohols. Kinet Catal 47, 834–845 (2006). https://doi.org/10.1134/S0023158406060048
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0023158406060048