Abstract
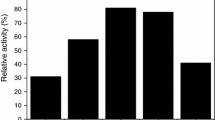
Thirty (5–40)% WO3/MO2 (M = Zr, Ti, Sn), heterogeneous acidic catalysts have been synthesized by two methods, specifically, via homogeneous acid solutions and from solutions brought to pH 9 with ammonia, both followed by calcination at 600–900°C. The catalysts have been characterized by IR spectroscopy and scanning electron microscopy, and their aqueous washings have been analyzed. Their acidity has been determined by the thermal analysis of samples containing adsorbed pyridine, and in terms of the proton affinity scale. Catalytic activities have been compared for cumene hydroperoxide (CHP) decomposition at 40°C in cumene and acetone. For all M, the catalysts are one type and contain W in strongly and weakly bound states, the latter being a polyoxometalate that can be washed off. Both tungstate phases are active in acid catalysis. Brønsted acid sites with a broad strength distribution have been found. The strongest of them are heteropolyacid protons. The catalysts 30% WO3/SnO2 and 20% WO3/ZrO2 (in acetone) and 10–20% WO3/TiO2 (in cumene) are the most active in CHP decomposition, and their activity is not related to their total acidity. Phases containing W6+ that form during the high-temperature synthesis are responsible for the high acidity, and additional protons that may appear owing to W6+ reduction can play only a minor role.
Similar content being viewed by others
References
Tanabe, K. and Holderich, W.F., Appl. Catal., A, 1999, vol. 181, no. 2, p. 399.
Weitkamp, J. and Traa, Y., Catal. Today, 1999, vol. 49, p. 193.
Aliev, E.A. and Adzhamov, K.Yu., Azerb. Khim. Zh., 1984, no. 5, p. 6.
Arata, K. and Hino, M., Proc. 10th Int. Congr. on Catalysis, Budapest, 1992.
Vaudagna, S.R., Canovese, S.A., Comelli, R.A., and Figoli, N.S., Appl. Catal., A 1998, vol. 168, no. 1, p. 93.
Barton, D.G., Soled, S.L., and Iglesia, E., Top. Catal., 1998, vol. 6, p. 87.
Maksimov, G.M., Fedotov, M.A., Bogdanov, S.V., et al., J. Mol. Catal., A: Chem., 2000, vol. 158, no. 1, p. 435.
Gutierrez-Alejandre, A., Castillo, P., Ramirez, J., et al., Appl. Catal., A, 2001, vol. 216, p. 181.
Greish, A.A., Demygin, S.S., and Kustov, L.M., Katal. Prom-sti, 2002, no. 6, p. 27.
Kuba, S., Lukinskas, P., Grasselli, R.K., et al., J. Catal., 2003, vol. 216, nos. 1–2, p. 353.
Chu, W., Echizen, T., Kamiya, Y., and Okuhara, T., Appl. Catal., A, 2004, vol. 259, no. 2, p. 199.
Kruzhalov, B.D. and Golovanenko, B.I., Sovmestnoe poluchenie fenola i atsetona (Simultaneous Preparation of Phenol and Acetone), Moscow: Goskhimizdat, 1963.
Maksimov, G.M., Maksimovskaya, R.I., and Kozhevnikov, I.V., Zh. Neorg. Khim., 1994, vol. 39, no. 4, p. 623.
Maksimov, G.M., Paukshtis, E.A., Budneva, A.A., et al., Izv. Akad. Nauk, Ser. Khim., 2001, no. 4, p. 563.
Eibl, S., Gates, B.C., and Knoezinger, H., Langmuir, 2001, vol. 17, no. 1, p. 107.
Ferraris, G., DeRossi, S., Gazzoli, D., et al., Appl. Catal., A, 2003, vol. 240, p. 119.
Pope, M.T., Heteropoly and Isopoly Oxometalates, Berlin: Springer, 1983.
Maksimov, G.M., Usp. Khim., 1995, vol. 64, no. 5, p. 480.
Boyse, R.A. and Ko, E.J., J. Catal., 1997, vol. 171, no. 1, p. 191.
Kazanskii, L.P. and Golubev, A.M., Khimiya soedinenii Mo(VI) i W(VI) (Chemistry of Mo(VI) and W(VI) Compounds), Novosibirsk, 1979.
Ramis, G., Busca, G., Cristiani, C., et al., Langmuir, 1992, vol. 8, no. 7, p. 1744.
Armendariz, H., Cortes, M.A., Hernandez, I., et al., J. Mater. Chem., 2003, vol. 13, no. 1, p. 143.
Triwahyono, S., Yamada, T., and Hattori, H., Appl. Catal., A, 2003, vol. 242, no. 1, p. 101.
Gorte, R.J., Catal. Lett., 1999, vol. 62, no. 1, p. 1.
Auroux, A., Top. Catal., 2002, vol. 19, nos. 3–4, p. 205.
Arena, F., Dario, R., and Parmaliana, A., Appl. Catal., A, 1998, vol. 170, no. 1, p. 127.
Srinivasan, R., Keogh, R.A., and Davis, B.H., Catal. Lett., 1996, vol. 36, nos. 1–2, p. 51.
Cai, H., Du, D., Ni, J., et al., Thermochim. Acta, 1997, vol. 292, nos. 1–2, p. 45.
Farcusiu, D., Catal. lett, 2001, vol. 71, nos. 1–2, p. 95.
Vartuli, J.C., Santiesteban, J.G., Traverso, P., et al., J. Catal., 1999, vol. 187, no. 1, p. 131.
Kuznetsova, L.I., Maksimov, G.M., and Likholobov, V.A., Kinet. Katal., 1999, vol. 40, no. 5, p. 688 [Kinet. Catal. (Engl. Transl.), vol. 40, no. 5, p. 622].
Kabachnik, M.I., Usp. Khim., 1979, vol. 48, no. 9, p. 1523.
Farcusiu, D. and Hancu, D., Catal. Lett., 1998, vol. 53, nos. 1–2, p. 3.
Kazansky, V.B., Catal. Today, 2002, vol. 73, nos. 1–2, p. 127.
Zakoshanskii, V.M., Katal. Prom-sti, 2004, no. 5, p. 3.
Zecchina, A., Lamberti, C., and Bordiga, S., Catal. Today, 1998, vol. 41, nos. 1–3, p. 169.
Hajiivanov, K., Lukinskas, P., and Knozinger, H., Catal. Lett., 2002, vol. 82, nos. 1–2, p. 73.
Baertsch, C.D., Komala, K.T., Chua, Y.H., and Iglesia, E., J. Catal., 2002, vol. 205, no. 1, p. 44.
Shimizu, K., Venkatraman, T.N., and Song, W., Appl. Catal., A, 2002, vol. 225, nos. 1–2, p. 33.
Author information
Authors and Affiliations
Additional information
Original Russian Text © G.M. Maksimov, G.S. Litvak, A.A. Budneva, E.A. Paukshtis, A.N. Salanov, V.A. Likholobov, 2006, published in Kinetika i Kataliz, 2006, Vol. 47, No. 4, pp. 581–588.
Rights and permissions
About this article
Cite this article
Maksimov, G.M., Litvak, G.S., Budneva, A.A. et al. WO3/MO2 (M = Zr, Sn, Ti) heterogeneous acid catalysts: Synthesis, study, and use in cumene hydroperoxide decomposition. Kinet Catal 47, 564–571 (2006). https://doi.org/10.1134/S0023158406040124
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0023158406040124