Abstract
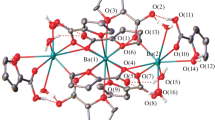
A coordination polymer with the [Ba5(fur)10(H2O)4]n composition (1) is prepared by the reaction of barium(II) acetate and 2-furancarboxylic acid (pyrosmucus, Hfur) in an aqueous alcoholic solution. The XRD data show that 1 contains three symmetrically independent Ba2+ cations with different coordinations of fur– anions and different coordination numbers. All three oxygen atoms of the furoate anions are connected by chelate and bridging coordination bonds with cations to form a complex honeycomb 3D framework. The simultaneous thermal analysis (STA) shows that high thermal stability of 1 is due to the network of Ba2+ cations formed by alternating bi- and trinuclear linear fragments. Dehydration of coordinated water molecules from the composition of 1 does not cause the framework destruction up to 400 °C.







Similar content being viewed by others
REFERENCES
A. K. D. Dimé, H. Cattey, D. Lucas, and C. H. Devillers. Electrosynthesis and X-ray crystallographic structure of ZnII meso-triaryltriphenylphosphonium porphyrin and structural comparison with MgII meso-triphenylphosphonium porphine. Eur. J. Inorg. Chem., 2018, 2018(44), 4834-4841. https://doi.org/10.1002/ejic.201801142
J. Bhattacharjee, A. Harinath, A. Sarkar, and T. K. Panda. Polymerization of ϵ-caprolactam to nylon-6 catalyzed by barium σ-borane complex under mild condition. ChemCatChem, 2019, 11(15), 3366-3370. https://doi.org/10.1002/cctc.201900920
S. Nandi, P. De Luna, R. Maity, D. Chakraborty, T. Daff, T. Burns, T. K. Woo, and R. Vaidhyanathan. Imparting gas selective and pressure dependent porosity into a non-porous solid via coordination flexibility. Mater. Horizons, 2019, 6(9), 1883-1891. https://doi.org/10.1039/c9mh00133f
B. Paluchowska, J. K. Maurin, and J. Leciejewicz. Direct and outer-sphere coordination of the magnesium ions in the crystal structures of complexes with 2-furancarboxylic acid (I) and 3-furancarboxylic acid (II). J. Chem. Crystallogr., 1997, 27(3), 177-182. https://doi.org/10.1007/bf02575986
J. Yang, X. Yin, L. Wu, J. Wu, J. Zhang, and M. Gozin. Alkaline and earth alkaline energetic materials based on a versatile and multifunctional 1-aminotetrazol-5-one ligand. Inorg. Chem., 2018, 57(24), 15105-15111. https://doi.org/10.1021/acs.inorgchem.8b02183
K. Wan, J. Yu, Q. Yang, and J. Xu. 5,5′-(1,4-Dioxo-1,2,3,4-tetrahydrophthalazine-6,7-diyl)bis(oxy)diisophthalate-based coordination polymers and their TNP sensing ability. Eur. J. Inorg. Chem., 2019, 2019(26), 3094-3102. https://doi.org/10.1002/ejic.201900558
H. Roueindeji, A. Ratsifitahina, T. Roisnel, V. Dorcet, S. Kahlal, J. Saillard, J. Carpentier, and Y. Sarazin. Metal⋯F–C bonding in low-coordinate alkaline earth fluoroarylamides. Chem. - Eur. J., 2019, chem.201901262. https://doi.org/10.1002/chem.201901262
T. Maity, D. Saha, S. Das, and S. Koner. Barium carboxylate metal–organic framework - synthesis, X-ray crystal structure, photoluminescence and catalytic study. Eur. J. Inorg. Chem., 2012, 2012(30), 4914-4920. https://doi.org/10.1002/ejic.201200417
X. Xu, F. Hu, and Q. Shuai. Facile synthesis, crystal structure and bioactivity evaluation of two novel barium complexes based on 2,4,6-trichlorophenoxyacetic acid and o-ferrocenylcarbonyl benzoic acid. New J. Chem., 2017, 41(22), 13319-13326. https://doi.org/10.1039/c7nj03046k
W. Su, Y. Shi, X. Hao, Z. Wang, Z. Du, Q. Li, and G. Yang. Synthesis, crystal structure and thermal behavior of two Ba(II) compounds derived from tetrazole-carboxylate ligands. Inorg. Chim. Acta, 2019, 490, 29-34. https://doi.org/10.1016/j.ica.2019.02.037
F. Liu, Y. Xu, L. Zhao, L. Zhang, W. Guo, R. Wang, and D. Sun. Porous barium–organic frameworks with highly efficient catalytic capacity and fluorescence sensing ability. J. Mater. Chem. A, 2015, 3(43), 21545-21552. https://doi.org/10.1039/c5ta03680a
L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne, and J. T. Hupp. Metal–organic framework materials as chemical sensors. Chem. Rev., 2012, 112(2), 1105-1125. https://doi.org/10.1021/cr200324t
J.-H. Wang, M. Li, and D. Li. A dynamic, luminescent and entangled MOF as a qualitative sensor for volatile organic solvents and a quantitative monitor for acetonitrile vapour. Chem. Sci., 2013, 4(4), 1793. https://doi.org/10.1039/c3sc00016h
J. Xiao, Y. Wu, M. Li, B.-Y. Liu, X.-C. Huang, and D. Li. Crystalline structural intermediates of a breathing metal-organic framework that functions as a luminescent sensor and gas reservoir. Chem. - Eur. J., 2013, 19(6), 1891-1895. https://doi.org/10.1002/chem.201203515
B. A. Fowler, G. F. Nordberg, M. Nordberg, and L. T. Friberg. Handbook on the Toxicology of Metals. Academic Press, 2011, 410.
P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris, and C. Serre. Metal–organic frameworks in biomedicine. Chem. Rev., 2012, 112(2), 1232-1268. https://doi.org/10.1021/cr200256v
P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regí, M. Sebban, F. Taulelle, and G. Férey. Flexible porous metal-organic frameworks for a controlled drug delivery. J. Am. Chem. Soc., 2008, 130(21), 6774-6780. https://doi.org/10.1021/ja710973k
E. Bartolomé, J. Bartolomé, S. Melnic, D. Prodius, S. Shova, A. Arauzo, J. Luzón, L. Badía-Romano, F. Luis, and C. Turta. Magnetic relaxation versus 3D long-range ordering in {Dy2Ba(α-fur)8}n furoate polymers. Dalton Trans., 2014, 43(28), 10999-11013. https://doi.org/10.1039/c4dt00538d
P. H. Bhargao and B. R. Srinivasan. Synthesis and structural characterization of a barium coordination polymer based on a μ2-monoatomic bridging 4-nitrobenzoate. J. Coord. Chem., 2019, 72(15), 2599-2615. https://doi.org/10.1080/00958972.2019.1666980
L. Krause, R. Herbst-Irmer, G. M. Sheldrick, and D. Stalke. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr., 2015, 48(1), 3-10. https://doi.org/10.1107/s1600576714022985
G. M. Sheldrick. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053229614024218
M. E. Nikiforova, I. A. Lutsenko, M. A. Kiskin, Y. V. Nelyubina, P. V. Primakov, O. B. Bekker, A. V. Khoroshilov, and I. L. Eremenko. Coordination polymer of Ba2+ with 2-furoic acid anions: Synthesis, structure, and thermal properties. Russ. J. Inorg. Chem., 2021, 66(9), 1343-1349. https://doi.org/10.1134/s0036023621090102
R. D. Hancock, C. J. Siddons, K. A. Oscarson, and J. M. Reibenspies. The structure of the 11-coordinate barium complex of the pendant-donor macrocycle 1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane: an analysis of the coordination numbers of barium(II) in its complexes. Inorg. Chim. Acta, 2004, 357(3), 723-727. https://doi.org/10.1016/j.ica.2003.06.016
C. Walbaum, I. Pantenburg, and G. Meyer. Penta-, hepta- und oktaiodid-anionen in salzen mit erdalkalimetall-kronenether-kationen / penta-, hepta-, and octaiodide anions in salts with alkaline earth metal crown ether cations. Z. Naturforsch. B, 2010, 65(9), 1077-1083. https://doi.org/10.1515/znb-2010-0904
R. E. Cramer, K. A. Mitchell, A. Y. Hirazumi, and S. L. Smith. Crystal structures of [Pb(NO3)6]4– and [Ba(NO3)6]4– salts of 24-pyrimidinium crown 6 {5,12,19,26,33,40-hexaamino-3,10,17,24,31,38-hexamethyl [1.6](1,5)pyrimidiniophane}. J. Chem. Soc., Dalton Trans., 1994, (4), 563. https://doi.org/10.1039/dt9940000563
E. Bartolomé, A. Arauzo, J. Luzón, S. Melnic, S. Shova, D. Prodius, J. Bartolomé, A. Amann, M. Nallaiyan, and S. Spagna. Slow relaxation in a {Tb2Ba(α-fur)8}n polymer with Ln = Tb(III) non-Kramers ions. Dalton Trans., 2019, 48(15), 5022-5034. https://doi.org/10.1039/c8dt05044a
B. Paluchowska, J. K. Maurin, and J. Leciejewicz. Variable coordination of barium in its complexes: Crystal structures of barium compounds with 2- and 3-furancarboxylic acids. Pol. J. Chem., 1996, 70(11), 1402-1410.
B. Paluchowska, J. K. Maurin, and J. Leciejewicz. Carboxylate and furan-ring oxygen bonded to calcium in polymeric calcium furoate. Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1996, 52(2), 347-351. https://doi.org/10.1107/s0108270195009553
B. Paluchowska, J. K. Maurin, and J. Leciejewicz. Hetero-ring oxygen coordination to strontium in strontium bis(2-furancarboxylate). Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1997, 53(3), 287-289. https://doi.org/10.1107/s0108270196011018
M. A. Uvarova, I. A. Lutsenko, M. E. Nikiforova, Y. V. Nelyubina, P. V. Primakov, A. V. Khoroshilov, M. A. Kiskin, and I. L. Eremenko. Gd(III) and Sm(III) 1D coordination polymers with 2-furoic acid: synthesis, structures, and thermal behavior. Russ. J. Coord. Chem., 2022, 48(8), 457-463. https://doi.org/10.1134/s1070328422080073
M. A. Uvarova, I. A. Lutsenko, M. A. Kiskin, Y. V. Nelyubina, P. V. Primakov, K. A. Babeshkin, N. N. Efimov, A. S. Goloveshkin, M. A. Shmelev, A. V. Khoroshilov, E. M. Zueva, M. M. Petrova, O. B. Bekker, and I. L. Eremenko. Nickel(II) complexes with 2-Hfur and N-donors: The magnetic effects of the structural variations, thermal properties and antimycobacterial activity against Mycolicibacterium smegmatis. Polyhedron, 2021, 203, 115241. https://doi.org/10.1016/j.poly.2021.115241
K. A. Koshenskova, I. A. Lutsenko, Y. V. Nelyubina, P. V. Primakov, T. M. Aliev, O. B. Bekker, A. V. Khoroshilov, S. N. Mantrov, M. A. Kiskin, and I. L. Eremenko. Copper(II) complexes with 5-nitro-2-furoic acid: Synthesis, structure, thermal properties, and biological activity. Russ. J. Inorg. Chem., 2022, 67(10), 1545-1556. https://doi.org/10.1134/s003602362270005x
I. A. Lutsenko, D. E. Baravikov, M. A. Kiskin, Y. V. Nelyubina, P. V. Primakov, O. B. Bekker, A. V. Khoroshilov, A. A. Sidorov, and I. L. Eremenko. Bioisostere modifications of Cu2+ and Zn2+ with pyromucic acid anions and N-donors: synthesis, structures, thermal properties, and biological activity. Russ. J. Coord. Chem., 2020, 46(6), 411-419. https://doi.org/10.1134/s1070328420060056
S. F. Chabira and M. Sebaa. Effect of thermal stabilizers (Ba/Cd/Zn metal salts carboxylate and dibasic lead stearate), on the photodegradation of PVC films. Rev. Sci. Technol., Synth., 2012, 24, 44.
Funding
The work was conducted out within the Russian Science Foundation project No. 22-13-00175.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 5, 110199.https://doi.org/10.26902/JSC_id110199
Rights and permissions
About this article
Cite this article
Nikiforova, M.E., Lutsenko, I.A., Dolgushin, F.M. et al. Honeycomb 3D Coordination Barium Polymer with 2-Furancarboxylic Acid Anions: Effect of Thermal Stability. J Struct Chem 64, 884–894 (2023). https://doi.org/10.1134/S0022476623050074
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623050074