Abstract
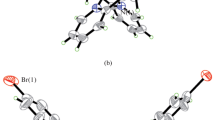
Two new zinc(II) and nickel(II) complexes, [Zn2L(μ2-η1:η1-CH3COO)(μ2-η1:η2-CH3COO)]n (1) and [Ni2(HL)2(H2O)2(μ1,3-N3)]Cl·2H2O (2·2H2O), where L is the doubly deprotonated form of 4,4′-dimethoxy-2,2′-(propane-1,3-diyldiiminodimethylene)diphenol (H2L), were synthesized and characterized by elemental analyses, infrared and electronic spectroscopy, as well as single crystal X-ray diffraction. Complex 1 is a bidentate bridging and chelating bridging acetate bridged polymeric zinc complex. In the asymmetric unit of the complex, one Zn atom is in distorted trigonal bipyramidal coordination, and the other one is in octahedral coordination. Complex 2·2H2O is an end-to-end azide bridged dinuclear nickel complex. The Ni atoms are in octahedral coordination. The inhibitory property on Jack bean urease of the complexes was studied, and the nickel complex has effective activity.







Similar content being viewed by others
REFERENCES
G. Mohiuddin, K. M. Khan, U. Salar, Kanwal, M. A. Lodhi, A. Wadood, M. Riaz, and S. Perveen. Bioorg. Chem., 2019, 83, 29-46. https://doi.org/10.1016/j.bioorg.2018.10.021
W.-Q. Song, M.-L. Liu, S.-Y. Li, and Z.-P. Xiao. Curr. Top. Med. Chem., 2022, 22, 95-107. https://doi.org/10.2174/1568026621666211129095441
Q. Liu, W.-W. Ni, Z. Li, C.-F. Bai, D.-D. Tan, C.-J. Pu, D. Zhou, Q.-P. Tian, N. Luo, K.-L. Tan, L. Dai, Y. Yan, Y. Pei, X.-H. Li, Z.-P. Xiao, and H.-L. Zhu. Eur. J. Pharm. Sci., 2018, 121, 293-300. https://doi.org/10.1016/j.ejps.2018.05.029
F. S. Carlos, R. J. Kunde, R. O. de Sousa, C. Weinert, A. D. Ulguim, F. Viero, I. Rossi, M. P. Buchain, C. L. Boechat, and F. A. D. Camargo. Nutr. Cycling Agroecosyst., 2022, 122, 313-324. https://doi.org/10.1007/s10705-022-10203-7
T. Lan, X. Q. He, Q. Wang, O. P. Deng, W. Zhou, L. Luo, G. D. Chen, J. Zeng, S. Yuan, M. Zeng, H. H. Xiao, and X. S. Gao. Appl. Soil Ecol., 2022, 174, 104412. https://doi.org/10.1016/j.apsoil.2022.104412
L. A. R. Ferreira, S. R. Silva, and O. T. Kolln. Inter. J. Plant Prod., 2022, 16, 313-328. https://doi.org/10.1007/s42106-022-00191-7
Q. Liu, W.-K. Shi, S.-Z. Ren, W.-W. Ni, W.-Y. Li, H.-M. Chen, P. Liu, J. Yuan, X.-S. He, J.-J. Liu, P. Cao, P.-Z. Yang, Z.-P. Xiao, and H.-L. Zhu. Eur. J. Med. Chem., 2018, 156, 126-136. https://doi.org/10.1016/j.ejmech.2018.06.065
W.-W. Ni, Q. Liu, S.-Z. Ren, W.-Y. Li, L.-L. Yi, H. Jing, L.-X. Sheng, Q. Wan, P.-F. Zhong, H.-L. Fang, H. Ouyang, Z.-P. Xiao, and H.-L. Zhu. Bioorg. Med. Chem., 2018, 26, 4145-4152. https://doi.org/10.1016/j.bmc.2018.07.003
W.-K. Shi, R.-C. Deng, P.-F. Wang, Q.-Q. Yue, Q. Liu, K.-L. Ding, M.-H. Yang, H.-Y. Zhang, S.-H. Gong, M. Deng, W.-R. Liu, Q.-J. Feng, Z.-P. Xiao, and H.-L. Zhu. Bioorg. Med. Chem., 2016, 24, 4519-4527. https://doi.org/10.1016/j.bmc.2016.07.052
A. F. Uberti, N. Callai-Silva, M. V. C. Grahl, A. R. Piovesan, E. G. Nachtigall, C. R. G. Furini, and C. R. Carlini. Inter. J. Mol. Sci., 2022, 23, 3091. https://doi.org/10.3390/ijms23063091
M.-L. Liu, W.-Y. Li, H.-L. Fang, Y.-X. Ye, S.-Y. Li, W.-Q. Song, Z.-P. Xiao, H. Ouyang, and H.-L. Zhu. ChemMedChem, 2022, 17, e202100618. https://doi.org/10.1002/cmdc.202100618
W.-W. Ni, H.-L. Fang, Y.-X. Ye, W.-Y. Li, L. Liu, Z.-J. Fu, Dawalamu, W.-Y. Zhu, K. Li, F. Li, X. Zou, H. Ouyang, Z.-P. Xiao, and H.-L. Zhu. Med. Chem., 2021, 17, 1046-1059. https://doi.org/10.2174/1573406416999200818152440
W.-Y. Li, W.-W. Ni, Y.-X. Ye, H.-L. Fang, X.-M. Pan, J.-L. He, T.-L. Zhou, J. Yi, S.-S. Liu, M. Zhou, Z.-P. Xiao, and H.-L. Zhu. J. Enzyme Inhib. Med. Chem., 2020, 35, 404-413. https://doi.org/10.1080/14756366.2019.1706503
W.-W. Ni, H.-L. Fang, Y.-X. Ye, W.-Y. Li, C.-P. Yuan, D.-D. Li, S.-J. Mao, S.-E. Li, Q.-H. Zhu, H. Ouyang, Z.-P. Xiao, and H.-L. Zhu. Future Med. Chem., 2020, 12, 1633-1645. https://doi.org/10.4155/fmc-2020-0048
Z.-P. Xiao, W.-K. Shi, P.-F. Wang, W. Wei, X.-T. Zeng, J.-R. Zhang, N. Zhu, M. Peng, B. Peng, X.-Y. Lin, H. Ouyang, X.-C. Peng, G.-C. Wang, and H.-L. Zhu. Bioorg. Med. Chem., 2015, 23, 4508-4513. https://doi.org/10.1016/j.bmc.2015.06.014
J. Ceramella, D. Iacopetta, A. Catalano, F. Cirillo, R. Lappano, and M. S. Sinicropi. Antibiotics (Basel, Switz.), 2022, 11, 191. https://doi.org/10.3390/antibiotics11020191
N. Lolak, M. Boga, G. D. Sonmez, M. Tuneg, A. Dogan, and S. Akocak. Pharm. Chem. J., 2022, 55, 1338-1344. https://doi.org/10.1007/s11094-022-02581-7
K. Rafiq, M. Khan, N. Muhammed, A. Khan, N. U. Rehman, B. E. M. Al-Yahyaei, M. Khiat, S. A. Halim, Z. R. Shah, and R. Csuk. Med. Chem. Res., 2021, 30, 712-728. https://doi.org/10.1007/s00044-020-02696-0
C.-H. Dai and F.-L. Mao. J. Struct. Chem., 2013, 54, 624-629. https://doi.org/10.1134/S0022476613030244
S. Thalamuthu and M. A. Neelakantan. Inorg. Chim. Acta, 2021, 516, 120109. https://doi.org/10.1016/j.ica.2020.120109
A. Sudha and S. J. A. Ali. Inorg. Chim. Acta, 2022, 534, 120817. https://doi.org/10.1016/j.ica.2022.120817
H. Wang, T. X. Lan, X. Zhang, D. M. Zhang, C. F. Bi, and Y. H. Fan. J. Inorg. Biochem., 2016, 165, 18-24. https://doi.org/10.1016/j.jinorgbio.2016.10.006
A. de Fatima, C. D. Pereira, C. R. S. D. G. Olimpio, B. G. D. Oliveira, L. L. Franco, and P. H. C. da Silva. J. Adv. Res., 2018, 13, 113-126. https://doi.org/10.1016/j.jare.2018.03.007
Y. M. Li, L. Y. Xu, M. M. Duan, B. T. Zhang, Y. H. Wang, Y. X. Guan, J. H. Wu, C. L. Jing, and Y. L. You. Polyhedron, 2019, 166, 146-152. https://doi.org/10.1016/j.poly.2019.03.051
H. Wang, C. G. Xu, X. Zhang, D. M. Zhang, F. Jin, and Y. H. Fan. J. Inorg. Biochem., 2020, 204, 110959. https://doi.org/10.1016/j.jinorgbio.2019.110959
M. Wozniczka, M. Lichawska, M. Sutradhar, M. Chmiela, W. Gonciarz, and M. Pajak. Pharmaceuticals, 2021, 14, 1254. https://doi.org/10.3390/ph14121254
S. Belaid, O. Benali-Baitich, G. Bouet, and A. Landreau. Chem. Pap., 2015, 69, 1350-1360. https://doi.org/10.1515/chempap-2015-0132
M. M. Duan, Y. M. Li, L. Y. Xu, H. L. Yang, F. W. Luo, Y. X. Guan, B. T. Zhang, C. L. Jing, and Z. L. You. Inorg. Chem. Commun., 2019, 100, 27-31. https://doi.org/10.1016/j.inoche.2018.12.009
D. S. Nesterov and O. V. Nesterova. Catalysts, 2021, 11, 1148. https://doi.org/10.3390/catal11101148
Y. Isaka, K. Oyama, Y. Yamada, T. Suenobu, and S. Fukuzumi. Catal. Sci. Technol., 2016, 6, 681-684. https://doi.org/10.1039/C5CY01845E
A. Paul, A. Figuerola, H. Puschmann, and S. C. Manna. Polyhedron, 2019, 157, 39-48. https://doi.org/10.1016/j.poly.2018.09.023
Saswati, M. Mohanty, A. Banerjee, S. Biswal, A. Horn, G. Schenk, K. Brzezinski, E. Sinn, H. Reuter, and R. Dinda. J. Inorg. Biochem., 2020, 203, 110908. https://doi.org/10.1016/j.jinorgbio.2019.110908
V. G. Vlasenko, A. S. Burlov, Y. V. Koshchienko, A. A. Kolodina, B. V. Chaltsev, Y. V. Zubavichus, V. N. Khrustalev, T. N. Danilenko, A. A. Zubenko, L. N. Fetisov, and A. I. Klimenko. Inorg. Chim. Acta, 2021, 523, 120408. https://doi.org/10.1016/j.ica.2021.120408
E. S. Koumousi, G. Lazari, S. Grammatikopoulos, C. Papatriantafyllopoulou, M. J. Manos, S. P. Perlepes, A. J. Tasiopoulos, G. Christou, and T. C. Stamatatos. Polyhedron, 2021, 206, 115298. https://doi.org/10.1016/j.poly.2021.115298
P.-J. Huang and H. Miyasaka. Dalton Trans., 2020, 49, 16970-16978. https://doi.org/10.1039/D0DT03615C
H. Jeon, J. Kim, J. Kim, K.-B. Cho, and S. Hong. Chem. Commun., 2022, 58, 4623-4626. https://doi.org/10.1039/D2CC01129H
J. Q. Wang, Y. Y. Luo, Y. X. Zhang, Y. Chen, F. Gao, Y. Ma, D. M. Xian, and Z. L. You. J. Coord. Chem., 2021, 74, 1028-1038. https://doi.org/10.1080/00958972.2020.1861603
A. Akay, C. Arici, O. Atakol, H. Fuess, and I. Svoboda. J. Coord. Chem., 2006, 59, 933-938. https://doi.org/10.1080/00958970500410374
A. Hazari, L. K. Das, R. M. Kadam, A. Bauza, A. Frontera, and A. Ghosh. Dalton Trans., 2015, 44, 3862-3876. https://doi.org/10.1039/C4DT03446E
B. Liu, J. Chai, S. Feng, and B. Yang. Spectrochim. Acta, Part A, 2015, 140, 437-443. https://doi.org/10.1016/j.saa.2015.01.012
Y. Song, P. Gamez, O. Roubeau, I. Mutikainen, U. Turpeinen, and J. Reedijk. Inorg. Chim. Acta, 2005, 358, 109-115. https://doi.org/10.1016/j.ica.2004.07.033
M. K. Taylor, J. Reglinski, L. E. A. Berlouis, and A. R. Kennedy. Inorg. Chim. Acta, 2006, 359, 2455-2464. https://doi.org/10.1016/j.ica.2006.01.039
Bruker, SMART and SAINT. Madison, WI: Bruker AXS Inc., 2002.
G. M. Sheldrick. SADABS. Göttingen, Germany: University of Göttingen, 1996.
G. M. Sheldrick. Acta Crystallogr., Sect. A, 2015, 71, 3-8. https://doi.org/10.1107/S2053273314026370
G. M. Sheldrick. Acta Crystallogr., Sect. C, 2015, 71, 3-8. https://doi.org/10.1107/S2053229614024218
J. Meletiadis, J. F. G. M. Meis, J. W. Mouton, J. P. Donnelly, and P. E. Verweij. J. Clin. Microbiol., 2000, 38, 2949-2954. https://doi.org/10.1128/JCM.38.8.2949-2954.2000
W. J. Geary. Coord. Chem. Rev., 1971, 7, 81-122. https://doi.org/10.1016/S0010-8545(00)80009-0
A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn, and G. C. Verschoor. J. Chem. Soc., Dalton Trans., 1984, 7, 1349-1356. https://doi.org/10.1039/DT9840001349
S. R. Korupoju, N. Mangayarkarasi, P. S. Zacharias, J. Mizuthani, and H. Nishihara. Inorg. Chem., 2002, 41, 4099-4101. https://doi.org/10.1021/ic0201102
V. K. Bhardwaj, M. S. Hundal, M. Corbella, V. Gomez, and G. Hundal. Polyhedron, 2012, 38, 224-234. https://doi.org/10.1016/j.poly.2012.03.029
M. Dey, C. P. Rao, P. K. Saarenketo, and K. Rissanen. Inorg. Chem. Commun., 2002, 5, 924-928. https://doi.org/10.1016/S1387-7003(02)00602-0
J. Reglinski, M. K. Taylor, and A. R. Kennedy. Inorg. Chem. Commun., 2006, 9, 736-739. https://doi.org/10.1016/j.inoche.2006.04.013
P. K. Bhaumik, K. Harms, and S. Chattopadhyay. Polyhedron, 2014, 68, 346-356. https://doi.org/10.1016/j.poly.2013.10.031
U. Kumar, J. Thomas, and N. Thirupathi. Inorg. Chem., 2010, 49, 62-72. https://doi.org/10.1021/ic901100z
Y. Luo, J. Wang, B. Zhang, Y. Guan, T. Yang, X. Li, L. Xu, J. Wang, and Z. You. J. Coord. Chem., 2020, 73, 1765-1777. https://doi.org/10.1080/00958972.2020.1795645
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 8, 99105.https://doi.org/10.26902/JSC_id99105
Rights and permissions
About this article
Cite this article
Jiang, J., Liu, B., Liu, Y. et al. SYNTHESES, CRYSTAL STRUCTURES AND UREASE INHIBITORY ACTIVITIES OF ZnII AND NiII COMPLEXES DERIVED FROM 4,4′-DIMETHOXY-2,2′-(PROPANE-1,3- DIYLDIIMINODIMETHYLENE)DIPHENOL. J Struct Chem 63, 1371–1381 (2022). https://doi.org/10.1134/S0022476622080182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622080182