Abstract
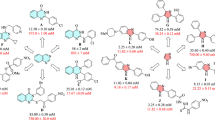
Combating pathological conditions related to hyperactivity of enzymes remains a formidable challenge for health. Small molecules therapy constitutes one of the means to circumvent the medical disorders resulting from enzyme hyperactivity. In this regard, we have synthesized structurally diverse amino acid hybrid Schiff bases (5a–5l and 10a–10k) and evaluated them for carbonic anhydrase II, α-glucosidase, and urease inhibitory potential. These new chemical scaffolds showed variable efficacies against the selected enzymes. The results indicated that compounds 5b (11.8 ± 1.33 µM), 10i (83.3 ± 1.13 µM), and 10f (88.2 ± 2.27 µM) are the most active scaffolds against carbonic anhydrase II, α-glucosidase, and urease, respectively. A structure–activity relationship revealed the most structural features contributing to the overall activities. Molecular docking suggested that these compounds possess excellent binding interactions with the active site residues of the targets by interacting through hydrogen bonding, π–π, and π–cation interactions.





Similar content being viewed by others
References
El-Faham A, El Massry AM, Amer A, Gohar YM. A versatile synthetic route to chiral quinoxaline derivatives from amino acids precursors. Lett Pept Sci. 2002;9:49–54.
Khattab SN. Synthesis and biological activity of novel amino acid-(N’-benzoyl) hydrazide and amino acid-(N’-nicotinoyl) hydrazide derivatives. Molecules. 2005;10:1218–28.
Marsham PR, Wardleworth JM, Boyle FT, Hennequin LF, Kimbell R, Brown M, et al. Design and synthesis of potent non-polyglutamatable quinazoline antifolate thymidylate synthase inhibitors. J Med Chem. 1999;42:3809–20.
Okada Y, Tsukatani M, Taguchi H, Yokoi T, Bryant SD, Lazarus LH. Amino acids and peptides. LII. Design and Synthesis of opioid mimetics containing a pyrazinone ring and examinaiton of their opioid receptor binding activity. Chem Pharm Bull. 1998;46:1374–82.
Polyak F, Lubell WD. Rigid dipeptide mimics: synthesis of enantiopure 5- and 7-benzyl and 5, 7-dibenzyl Indolizidinone amino acids via enolization and alkylation of δ-Oxo α, ω-Di-[N-(9-(9-phenylfluorenyl)) amino] azelate esters. J Org Chem. 1998;63:5937–47.
Sun G, Uretsky NJ, Wallace LJ, Shams G, Weinstein DM, Miller DD. Synthesis of chiral 1-(2′-amino-2′-carboxyethyl)-1, 4-dihydro-6, 7-quinoxaline-2, 3-diones: α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor agonists and antagonists. J Med Chem. 1996;39:4430–8.
Xia Y, Yang ZY, Xia P, Bastow KF, Nakanishi Y, Lee K-H. Antitumor agents. part 202: novel 2′-amino chalcones: design, synthesis and biological evaluation. Bioorg Med Chem Lett. 2000;10:699–701.
Barrett D, Tanaka A, Harada K, Ohki H, Watabe E, Maki K, et al. Synthesis and biological activity of novel macrocyclic antifungals: acylated conjugates of the ornithine moiety of the lipopeptidolactone FR901469. Bioorg Med Chem Lett. 2001;11:479–82.
Hughes AB, editor. Amino acids, peptides and proteins in organic chemistry. Weinheim: Wiley-VCH; 2009.
O’Donnell MJ. Benzophenone Schiff bases of glycine derivatives: versatile starting materials for the synthesis of amino acids and their derivatives. Tetrahedron. 2019;75:3667–96.
Zafar H, Ahmad A, Khan AU, Khan TA. Synthesis, characterization and antimicrobial studies of Schiff base complexes. J Mol Struct. 2015;1097:129–35.
Cheng K, Zheng QZ, Qian Y, Shi L, Zhao J, Zhu HL. Synthesis, antibacterial activities and molecular docking studies of peptide and Schiff bases as targeted antibiotics. Bioorg Med Chem. 2009;17:7861–71.
Jarrahpour A, Khalili D, De Clercq E, Salmi C, Brunel JM. Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules. 2007;12:1720–30.
Panchal PK, Pansuriya PB, Patel M. In-vitro biological evaluation of some ONS and NS donor Schiff’s bases and their metal complexes. J Enzym Inhib Med Chem. 2006;21:453–8.
Avupati VR, Yejella RP, Parala VR, Killari KN, Papasani VM, Cheepurupalli P, et al. Synthesis, characterization and in vitro biological evaluation of some novel 1, 3, 5-triazine–Schiff base conjugates as potential antimycobacterial agents. Bioorg Med Chem Lett. 2013;23:5968–70.
Sashidhara KV, Rosaiah JN, Bhatia G, Saxena J. Novel keto-enamine Schiffs bases from 7-hydroxy-4-methyl-2-oxo-2H-benzo [h] chromene-8, 10-dicarbaldehyde as potential antidyslipidemic and antioxidant agents. Eur J Med Chem. 2008;43:2592–6.
Vančo J, Švajlenová O, Račanská E, Muselík J, Valentová J. Antiradical activity of different copper (II) Schiff base complexes and their effect on alloxan-induced diabetes. J Trace Elem Med Bio. 2004;18:155–61.
Ambike V, Adsule S, Ahmed F, Wang Z, Afrasiabi Z, Sinn E, et al. Copper conjugates of nimesulide Schiff bases targeting VEGF, COX and Bcl-2 in pancreatic cancer cells. J Inorg Biochem. 2007;101:1517–24.
Gajula MR, Reddy YVR. Synthesis, characterization and in vitro biological evaluation of some new 1, 3, 5-triazine-bis-azomethine hybrid molecules as potential antitubercular agents. Eur J Chem. 2014;5:374–9.
Sinha D, Tiwari AK, Singh S, Shukla G, Mishra P, Chandra H, et al. Synthesis, characterization and biological activity of Schiff base analogues of indole-3-carboxaldehyde. Eur J Med Chem. 2008;43:160–5.
You ZL, Shi DH, Xu C, Zhang Q, Zhu HL. Schiff base transition metal complexes as novel inhibitors of xanthine oxidase. Eur J Med Chem. 2008;43:862–71.
Amin R, Krammer B, Abdel-Kader N, Verwanger T, El-Ansary A. Antibacterial effect of some benzopyrone derivatives. Eur J Med Chem. 2010;45:372–8.
Bharti S, Nath G, Tilak R, Singh S. Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2, 4-disubstituted thiazole ring. Eur J Med Chem. 2010;45:651–60.
Ye XX, Chen ZF, Zhang AJ, Zhang LX. Synthesis and biological evaluation of some novel Schiff’s bases from 1, 2, 4-triazole. Molecules. 2007;12:1202–9.
Gacche R, Gond D, Dhole N, Dawane B. Coumarin Schiff-bases: as antioxidant and possibly anti-inflammatory agents. J Enzym Inhib Med Chem. 2006;21:157–61.
Toyota E, Sekizaki H, Takahashi Y-U, Itoh K, Tanizawa K. Amidino-containing Schiff base copper (II) and iron (III) chelates as a thrombin inhibitor. Chem Pharm Bull. 2005;53:22–26.
Cardile V, Panico A, Geronikaki A, Gentile B, Ronsisvalle G. In vitro evaluation of thiazolyl and benzothiazolyl Schiff bases on pig cartilage. Il Farmaco. 2002;57:1009–13.
Doddareddy MR, Cho YS, Koh HY, Pae AN. CoMFA and CoMSIA 3D QSAR analysis on N1-arylsulfonylindole compounds as 5-HT6 antagonists. Bioorg Med Chem. 2004;12:3977–85.
Jayashree B, Anuradha D, Venugopala K. Synthesis and characterization of Schiff bases of 2’-amino-4’-(6-chloro-3-coumarinyl)-thiazole as potential NSAIDs. Asian J Chem. 2005;17:2093.
Bhandari SV, Bothara KG, Raut MK, Patil AA, Sarkate AP, Mokale VJ. Design, synthesis and evaluation of antiinflammatory, analgesic and ulcerogenicity studies of novel S-substituted phenacyl-1, 3, 4-oxadiazole-2-thiol and Schiff bases of diclofenac acid as nonulcerogenic derivatives. Bioorg Med Chem. 2008;16:1822–31.
D’Ascenzio M, Guglielmi P, Carradori S, Secci D, Florio R, Mollica A, et al. Open saccharin-based secondary sulfonamides as potent and selective inhibitors of cancer-related carbonic anhydrase IX and XII isoforms. J Enzym Inhib Med Chem. 2017;32:51–59.
Gokcen T, Gulcin I, Ozturk T, Goren AC. A class of sulfonamides as carbonic anhydrase I and II inhibitors. J Enzym Inhib Med Chem. 2016;31:180–8.
Gul HI, Mete E, Taslimi P, Gulcin I, Supuran CT. Synthesis, carbonic anhydrase I and II inhibition studies of the 1, 3, 5-trisubstituted-pyrazolines. J Enzym Inhib Med Chem. 2017;32:189–92.
Ho YT, Purohit A, Vicker N, Newman SP, Robinson JJ, Leese MP, et al. Inhibition of carbonic anhydrase II by steroidal and non-steroidal sulphamates. Biochem Biophys Res Commun. 2003;305:909–14.
Ibrahim DA, Lasheen DS, Zaky MY, Ibrahim AW, Vullo D, Ceruso M, et al. Design and synthesis of benzothiazole-6-sulfonamides acting as highly potent inhibitors of carbonic anhydrase isoforms I, II, IX and XII. Bioorg Med Chem. 2015;23:4989–99.
Akocak S, Lolak N, Nocentini A, Karakoc G, Tufan A, Supuran CT. Synthesis and biological evaluation of novel aromatic and heterocyclic bis-sulfonamide Schiff bases as carbonic anhydrase I, II, VII and IX inhibitors. Bioorg Med Chem. 2017;25:3093–7.
Sağlık BN, Çevik UA, Osmaniye D, Levent S, Çavuşoğlu BK, Demir Y, et al. Synthesis, molecular docking analysis and carbonic anhydrase I-II inhibitory evaluation of new sulfonamide derivatives. Bioorg Chem. 2019;91:103153.
Aggarwal M, Boone CD, Kondeti B, McKenna R. Structural annotation of human carbonic anhydrases. J Enzym Inhib Med Chem. 2013;28:267–77.
Supuran CT. Carbonic anhydrases-an overview. Curr Pharm Des. 2008;14:603–14.
Webb S, Sherratt J, Fish R. Mathematical modelling of tumor acidity: regulation of intracellular pH. J Theor Biol. 1999;196:237–50.
Lee AH, Tannock IF. Heterogeneity of intracellular pH and of mechanisms that regulate intracellular pH in populations of cultured cells. Cancer Res. 1998;58:1901–8.
Montcourrier P, Silver I, Farnoud R, Bird I, Rochefort H. Breast cancer cells have a high capacity to acidify extracellular milieu by a dual mechanism. Clin Exp Metastasis. 1997;15:382–92.
Frazier ML, Lilly BJ, Wu EF, Ota T, Hewett-Emmett D. Carbonic anhydrase II gene expression in cell lines from human pancreatic adenocarcinoma. Pancreas. 1990;5:507–14.
Parkkila AK, Herva R, Parkkila S, Rajaniemi H. Immunohistochemical demonstration of human carbonic anhydrase isoenzyme II in brain tumours. Histochem J. 1995;27:974–82.
Parkkila S, Rajaniemi H, Parkkila AK, Kivelä J, Waheed A, Pastoreková S, et al. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci USA. 2000;97:2220–4.
Achal V, Pan X. Characterization of urease and carbonic anhydrase producing bacteria and their role in calcite precipitation. Curr Microbiol. 2011;62:894–902.
Şentürk M, Gülçin İ, Beydemir Ş, Küfrevioğlu Öİ, Supuran CT. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des. 2011;77:494–9.
Khan KM, Wadood A, Ali M, Ul-Haq Z, Lodhi MA, Khan M, et al. Identification of potent urease inhibitors via ligand-and structure-based virtual screening and in vitro assays. J Mol Graph Model. 2010;28:792–8.
Qin Y, Cabral JM. Kinetic studies of the urease-catalyzed hydrolysis of urea in a buffer-free system. Appl Biochem Biotech. 1994;49:217–40.
Mobley H, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Mol Biol Rev. 1995;59:451–80.
Saleem M, Hareem S, Khan A, Naheed S, Raza M, Hussain R, et al. Dual inhibitors of urease and carbonic anhydrase-II from Iris species. Pure Appl Chem. 2019;91:1695–707.
Gao C, Li B, Zhang B, Sun Q, Li L, Li X, et al. Synthesis and biological evaluation of benzimidazole acridine derivatives as potential DNA-binding and apoptosis-inducing agents. Bioorg Med Chem. 2015;23:1800–7.
Gao H, Huang YN, Xu PY, Kawabata J. Inhibitory effect on α-glucosidase by the fruits of Terminalia chebula Retz. Food Chem. 2007;105:628–34.
Chiasson J-L. Prevention of type 2 diabetes: fact or fiction? Expert Opin Pharm. 2007;8:3147–58.
Rehman NU, Khan A, Al-Harrasia A, Hussain H, Wadood A, Riaz M, et al. New α-glucosidase inhibitors from the resins of Boswellia species with structure–glucosidase activity and molecular docking studies. Bioorg Chem. 2018;79:27–33.
Rehman NU, Khan A, Al-Harrasi A, Khiat M, Hidayat Hussain H, Wadood A, et al. Natural urease inhibitors from Aloe vera resin and Lycium shawii and their structural-activity relationship and molecular docking study. Bioorg Chem. 2019;88:102955.
Rehman N, Halim SA, Khan M, Hussain H, Yar Khan H, Khan A, et al. Antiproliferative and carbonic anhydrase II inhibitory potential of chemical constituents from Lycium shawii and aloe vera: evidence from in silico target fishing and in vitro testing. Pharmaceuticals. 2020;13:94.
Golmohammadi F, Balalaie S, Hamdan F, Maghari S. Efficient synthesis of novel conjugated 1, 3, 4-oxadiazole–peptides. N J Chem. 2018;42:4344–51.
Shank RP, Doose DR, Streeter AJ, Bialer M. Plasma and whole blood pharmacokinetics of topiramate: the role of carbonic anhydrase. Epilepsy Res. 2005;63:103–12.
Oki T, Matsui T, Osajima Y. Inhibitory effect of α-glucosidase inhibitors varies according to its origin. J Agric Food Chem. 1999;47:550–3.
Rehman NU, Rafiq K, Khan A, Ahsan Halim S, Ali L, Al-Saady N, et al. α-Glucosidase inhibition and molecular docking studies of natural brominated metabolites from marine macro brown alga Dictyopteris hoytii. Mar Drugs. 2019;17:666.
Acknowledgements
The authors are grateful to The Research Council (TRC), Oman, for funding through the project (BFP/RGP/CBS/18/011) and University of Nizwa, Oman, for the generous support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rafiq, K., Khan, M., Muhammed, N. et al. New amino acid clubbed Schiff bases inhibit carbonic anhydrase II, α-glucosidase, and urease enzymes: in silico and in vitro. Med Chem Res 30, 712–728 (2021). https://doi.org/10.1007/s00044-020-02696-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02696-0