Abstract
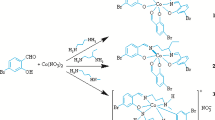
A mononuclear cobalt(III) complex [CoL(NCS)2(OH2)] (1) and a trinuclear cobalt(III–II–III) complex [Co{CoLN3(μ1,1-N3)2(CH3OH)}2] (2), derived from the Schiff base ligand 2-(((2-(pyrrolidin-1-yl)ethyl)imino)methyl)phenol (HL), are synthesized and characterized by IR and electronic spectra. The structures of both complexes are studied in detail by single crystal X-ray diffraction. In complex 1, the Co(III) atom is coordinated by three donor atoms of the Schiff base ligand, two thiocyanate N atoms, and one water O atom, forming an octahedral geometry. In complex 2, the terminal Co(III) atom is coordinated by three donor atoms of the Schiff base ligand, two end-on azide N atoms, and one terminal azide N atom, forming an octahedral geometry. The central Co(II) atom is coordinated by four end-on azide N atoms and two methanol O atoms, forming an octahedral geometry. The complexes exhibit interesting antibacterial activities against B. subtilis and E. coli.







Similar content being viewed by others
REFERENCES
R. Cordeiro and M. Kachroo. Bioorg. Med. Chem. Lett., 2020, 30, 127655. https://doi.org/10.1016/j.bmcl.2020.127655
Q. H. Weng, J. Q. Yi, X. P. Chen, D. W. Luo, Y. D. Wang, W. M. Sun, J. Kang, and Z. Z. Han. ACS Omega, 2020, 5, 24864-24870. https://doi.org/10.1021/acsomega.0c03591
K. Rafiq, M. Khan, N. Muhammed, A. Khan, N. U. Rehman, B. E. M. Al-Yahyaei, M. Khiat, S. A. Halim, Z. R. Shah, R. Csuk, and A. Al-Harrasi. Med. Chem. Res., 2021, 30, 712-728. https://doi.org/10.1007/s00044-020-02696-0
F. Naz, Kanwal, M. Latif, U. Salar, K. M. Khan, M. al-Rashida, I. Ali, B. Ali, M. Taha, and S. Perveen. Bioorg. Chem., 2020, 105, 104365. https://doi.org/10.1016/j.bioorg.2020.104365
Q. T. Nguyen, P. N. P. Thi, and V. T. Nguyen. Bioinorg. Chem. Appl., 2021, 2021, 6696344. https://doi.org/10.1155/2021/6696344
J. Kiriratnikom, N. Laiwattanapaisarn, K. Vongnam, N. Thavornsin, P. Sae-ung, S. Kaeothip, A. Euapermkiati, S. Namuangruk, and K. Phomphrai. Inorg. Chem., 2021, 60, 6147-6151. https://doi.org/10.1021/acs.inorgchem.0c03732
N. S. Abdel-Kader, H. Moustafa, A. L. El-Ansary, O. E. Sherif, and A. M. Farghaly. New J. Chem., 2021, 45, 7714-7730. https://doi.org/10.1039/D0NJ05688J
F. Aghvami, A. Ghaffari, M. Kucerahova, M. Dusek, R. Karimi-Nami, M. Amini, and M. Behzad. Polyhedron, 2021, 200, 115135. https://doi.org/10.1016/j.poly.2021.115135
B. Pinchaipat, T. Khudkham, S. Wongsuwan, R. Chotima, K. Chainok, and T. Pila. Mater. Lett., 2021, 293, 129749. https://doi.org/10.1016/j.matlet.2021.129749
T. A. Bazhenova, L. V. Zorina, S. V. Simonov, Y. V. Manakin, A. B. Kornev, K. A. Lyssenko, V. S. Mironov, I. F. Gilmutdinov, and E. B. Yagubskii. Inorg. Chim. Acta, 2021, 522, 120358. https://doi.org/10.1016/j.ica.2021.120358
T. K. Karmakar, M. Ghosh, M. Fleck, G. Pilet, and D. Bandyopadhyay. J. Coord. Chem., 2012, 65, 2612-2622. https://doi.org/10.1080/00958972.2012.700514
A. Banerjee, A. Guha, J. Adhikary, A. Khan, K. Manna, S. Dey, E. Zangrando, and D. Das. Polyhedron, 2013, 60, 102-109. https://doi.org/10.1016/j.poly.2013.05.014
M. Kalita, P. Gogoi, P. Barman, and B. Sarma. J. Coord. Chem., 2014, 67, 2445-2454. https://doi.org/10.1080/00958972.2014.946917
P. Pattanayak, J. L. Pratihar, D. Patra, P. Brandao, and V. Felix. Inorg. Chim. Acta, 2014, 418, 171-179. https://doi.org/10.1016/j.ica.2014.04.021
M. Hasanzadeh, M. Salehi, M. Kubicki, S. M. Shahcheragh, G. Dutkiewicz, M. Pyziak, and A. Khaleghian. Transition Met. Chem., 2014, 39, 623-632. https://doi.org/10.1007/s11243-014-9841-x
M. Ghosh, M. Layek, M. Fleck, R. Saha, and D. Bandyopadhyay. Polyhedron, 2015, 85, 312-319. https://doi.org/10.1016/j.poly.2014.08.014
A. Frei, A. P. King, G. J. Lowe, A. K. Cain, F. L. Short, H. Dinh, A. G. Elliott, J. Zuegg, J. J. Wilson, and M. A. T. Blaskovich. Chem. Eur. J., 2020, 27, 2021-2029. https://doi.org/10.1002/chem.202003545
M. Jafari, M. Salehi, M. Kubicki, A. Arab, and A. Khaleghian. Inorg. Chim. Acta, 2017, 462, 329-335. https://doi.org/10.1016/j.ica.2017.04.007
H. A. R. Pramanik, P. C. Paul, P. Mondal, and C. R. Bhattacharjee. J. Mol. Struct., 2015, 1100, 496-505. https://doi.org/10.1016/j.molstruc.2015.07.076
M. N. Ahamad, K. Iman, M. K. Raza, M. Kumar, A. Ansari, M. Ahmad, and M. Shahid. Bioorg. Chem., 2020, 95, 103561. https://doi.org/10.1016/j.bioorg.2019.103561
S. H. Rahaman, H. K. Fun, and B. K. Ghosh. Polyhedron, 2000, 24, 3091-3097. https://doi.org/10.1016/j.poly.2005.06.028
S. Naiya, C. Biswas, M. G. B. Drew, C. J. Gomez-Garcia, J. M. Clemente-Juan, and A. Ghosh. Inorg. Chem., 2010, 49, 6616-6627. https://doi.org/10.1021/ic1005456
G. Bhargavi, M. V. Rajasekharan, and J. P. Tuchagues. Inorg. Chim. Acta, 2009, 362, 3247-3252. https://doi.org/10.1016/j.ica.2009.02.032
P. Bhowmik, S. Chattopadhyay, M. G. B. Drew, C. Diaz, and A. Ghosh. Polyhedron, 2010, 29, 2637-2642. https://doi.org/10.1016/j.poly.2010.06.014
V. L. Gein, M. I. Kazantseva, L. I. Varkentin, T. M. Zamaraeva, A. N. Yankin, E. V. Beletskii, and V. V. Novikova. Russ. J. Gen. Chem., 2020, 90, 1426-1431. https://doi.org/10.1134/S1070363220080083
L.-S. Feng, M.-L. Liu, K. Lv, Y. Chai, S. Wang, J. Cao, and H.-Y. Guo. Asian J. Chem., 2013, 25, 2327/2328. https://doi.org/10.14233/ajchem.2013.13280
S. Haddad, S. Boudriga, T. N. Akhaja, J. P. Raval, F. Porzio, A. Soldera, M. Askri, M. Knorr, Y. Rousselin, M. M. Kubicki, and D. Rajani. New J. Chem., 2015, 39, 520-528. https://doi.org/10.1039/C4NJ01008F
A. S. H. Alsamarrai and S. S. Abdulghani. Molecules, 2021, 26, 533. https://doi.org/10.3390/molecules26030533
P. Prabhakaran, M. Subaraja, and P. Rajakumar. Chemistry Select, 2018, 3, 4687-4693. https://doi.org/10.1002/slct.201800033
J. Huang, H. T. Liu, M. L. Liu, R. Zhang, L. H. Li, B. Wang, M. H. Wang, C. L. Wang, and Y. Lu. Bioorg. Med. Chem. Lett., 2015, 25, 5058-5063. https://doi.org/10.1016/j.bmcl.2015.10.027
K. Lv, M.-L. Liu, L.-S. Feng, L.-Y. Sun, Y.-X. Sun, Z.-Q. Wei, and H.-Q. Guo. Eur. J. Med. Chem., 2012, 47, 619-625. https://doi.org/10.1016/j.ejmech.2011.10.048
E. Kocabas, A. B. Sariguney, F. Erci, R. Cakir-Koc, H. O. Kocabas, E. Torlak, and A. Coskun. Biointerface Res. Appl. Chem., 2021, 11, 12178-12185. https://doi.org/10.33263/BRIAC114.1217812185
XSCANS, Data Collection and Reduction Program, Version 2.2. Madison, WI: Siemens Analytical X-ray Instruments Inc., 1994.
G. M. Sheldrick. SADABS: Program for Empirical Absorption Correction of Area Detector Data. Göttingen, Germany: University of Göttingen, 1996.
G. M. Sheldrick. SHELXTL version 5.1: Program for the Solution and Refinement of Crystal Structures. Madison, WI, USA: Bruker AXS Inc., 1999.
G. M. Sheldrick. SHELXS/L-97: Programs for Crystal Structure Determination. Göttingen, Germany: University of Göttingen, 1997.
J. Meletiadis, J. Meis, J. W. Mouton, J. P. Donnelly, and P. E. Verweij. J. Clin. Microbiol., 2000, 38, 2949-2954. https://doi.org/10.1128/JCM.38.8.2949-2954.2000
J.-L. Hou, H.-Y. Wu, C.-B. Sun, Y. Bi, and W. Chen. Acta Chim. Slov., 2020, 67, 860-865. https://doi.org/10.17344/acsi.2020.5824
S. Banerjee, J.-T. Chen, and C.-Z. Lu. Polyhedron, 2007, 26, 686-694. https://doi.org/10.1016/j.poly.2006.08.035
M. Fleck, M. Layek, R. Saha, and D. Bandyopadhyay. Transition Met. Chem., 2013, 38, 715-724. https://doi.org/10.1007/s11243-013-9741-5
N. Mondal, D. K. Dey, S. Mitra, and K. M. A. Malik. Polyhedron, 2000, 19, 2707-2711. https://doi.org/10.1016/S0277-5387(00)00584-2
Y. Zhu and W.-H. Li. Transition Met. Chem., 2010, 35, 745-749. https://doi.org/10.1007/s11243-010-9388-4
A. Hazari, L. K. Das, R. M. Kadam, A. Bauza, A. Frontera, and A. Ghosh. Dalton Trans., 2015, 44, 3862-3876. https://doi.org/10.1039/C4DT03446E
A. Datta, K. Das, C. Sen, N. K. Karan, J.-H. Huang, C.-H. Lin, E. Garribba, C. Sinha, T. Askun, and P. Celikboyun. Spectrochim. Acta, Part A, 2015, 148, 427-434. https://doi.org/10.1016/j.saa.2015.04.014
M. Sarwar, A. M. Madalan, F. Lloret, M. Julve, and M. Andruh. Polyhedron, 2011, 30, 2414-2420. https://doi.org/10.1016/j.poly.2011.06.011
A. Ray, S. Banerjee, R. J. Butcher, C. Desplanches, and S. Mitra. Polyhedron, 2008, 27, 2409-2415. https://doi.org/10.1016/j.poly.2008.04.018
F. Luo, Y. Ning, M.-B. Luo, and G.-L. Huang. CrystEngComm, 2010, 12, 2769-2774. https://doi.org/10.1039/c000734j
L. Pogany, J. Moncol, M. Gal, I. Salitros, and R. Boca. Inorg. Chim. Acta, 2017, 462, 23-29. https://doi.org/10.1016/j.ica.2017.03.001
S. Banerjee, M. Nandy, S. Sen, S. Mandal, G. M. Rosair, A. M. Z. Slawin, C. J. Gomez Garcia, J. M. Clemente-Juan, E. Zangrando, N. Guidolin, and S. Mitra. Dalton Trans., 2011, 40, 1652-1661. https://doi.org/10.1039/c0dt00923g
Y.-M. Zhou, X.-R. Ye, F.-B. Xin, and X.-Q. Xin. Transition Met. Chem., 1999, 24, 118-120. https://doi.org/10.1023/A:1006989707001
A. Ray, D. Sadhukhan, G. M. Rosair, C. J. Gomez-Garcia, and S. Mitra. Polyhedron, 2009, 28, 3542-3550. https://doi.org/10.1016/j.poly.2009.07.017
R. W. Handel, H. Willms, G. B. Jameson, K. J. Berry, B. Moubaraki, K. S. Murray, and S. Brooker. Eur. J. Inorg. Chem., 2010, 3317-3327. https://doi.org/10.1002/ejic.201000288
Y.-L. Sang, X.-S. Lin, and W.-D. Sun. Acta Chim. Slov., 2016, 63, 856-863. http://dx.doi.org/10.17344/acsi.2016.2795
Y. M. Hao. Russ. J. Coord. Chem., 2018, 44, 45-51. https://doi.org/10.1134/S1070328418010050
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 2, pp. 105-108.https://doi.org/10.26902/JSC_id87107
Rights and permissions
About this article
Cite this article
Wen, X., Chen, W., Hou, J. et al. SYNTHESES, CHARACTERIZATION, AND CRYSTAL STRUCTURES OF COBALT(III) COMPLEXES DERIVED FROM 2-(((2- (PYRROLIDIN-1-YL)ETHYL)IMINO)METHYL) PHENOL WITH THE ANTIBACTERIAL ACTIVITY. J Struct Chem 63, 165–175 (2022). https://doi.org/10.1134/S0022476622020019
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622020019