Abstract
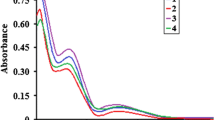
A new copper(I) heteroleptic pyridyl functionalized dithiocarbamate(dtc) complex, [Cu(L)2dppf]·2H2O·MeOH, (1) (where L = N-benzyl-N-methylpyridyldtc and dppf = = diphenyl phosphinoferrocene), has been synthesized from the reaction of [Cu2(μ-Br)2(k2-P,P-dppf)2] and dithiocarbamate ligand (L). The synthesized complex has been characterized by elemental analysis, spectroscopy techniques (IR, 1H, 13C, 31P NMR, and UV-Vis), and single-crystal X-ray crystallography. In this heteroleptic complex, the Cu atom forms distorted tetrahedral coordination geometry. The supramolecular architecture in the complex has been sustained in the solid phase by, C–H⋯O and C–H⋯π (chelate = CuS2C) interactions. The emission spectrum of the complex has been studied in DCM solution. The charge-transfer excited state is quenched due to intramolecular energy transfer from the {Cu(S,S)(P,P)} moiety to the ferrocene therefore dppf-based complex shows no detectable emission at room temperature. This complex is weakly conducting and exhibit semiconductor behavior at room temperature.








Similar content being viewed by others
REFERENCES
T. J. Kealy and P. L. Pauson. Nature, 1951, 168, 1039. https://doi.org/10.1038/1681039b0
L. R. Butler. Polyhedron, 1992, 11, 3117. https://doi.org/10.1016/S0277-5387(00)83651-7
A. Togni and T. Hayashi. Ferrocenes: Homogeneous Catalysis, Organic Synthesis, Materials Science. VCH Publishers, 1995. https://doi.org/10.1002/9783527615599
C. Valério, J. L. Fillaut, J. Ruiz, J. Guittard, J. C. Blais, and D. Astruc. J. Am. Chem. Soc., 1997, 119, 2588. https://doi.org/10.1021/ja964127t
P. D. Beer, P. A. Gale, and G. Z. Chen. J. Chem. Soc., Dalton Trans., 1999, 1897. https://doi.org/10.1039/a901462d
J. D. Carr, S. J. Coles, W. W. Hassan, M. B. Hursthouse, K. M. A. Malik, and H. R. Tucker. J. Chem. Soc., Dalton Trans., 1999, 57. https://doi.org/10.1039/a807453d
D. R. van Staveren and N. Metzler-Nolte. Chem. Rev., 2004, 104, 5931. https://doi.org/10.1021/cr0101510
G. Jaouen, S. Top, A. Vessières, and G. Jaouen. Bioorganometallics: Biomolecules, Labeling, Medicine. Germany: Wiley-VCH, 2006. https://doi.org/10.1002/3527607692
B. Lal, A. Badshah, A. A. Altaf, N. Khan, and S. Ullah. Appl. Organomet. Chem., 2011, 25, 843-855. https://doi.org/10.1002/aoc.1843
A. A. Altaf, M. Hamayun, B. Lal, M. N. Tahir, A.A. Holder, A. Badshah, and D. C. Crans. Dalton Trans., 2018, 47, 11769. https://doi.org/10.1039/C8DT01726C
D. Coucouvanis. Prog. Inorg. Chem., 1970, 11, 233.
D. Coucouvanis. Prog. Inorg. Chem., 1979, 26, 301.
S. R. Rao. Xanthates and Related Compounds. New York: Marcel Dekker, 1971.
G. Hogarth. Mini-Rev. Med. Chem., 2012, 12, 1202. https://doi.org/10.2174/138955712802762095
P. J. Heard. Prog. Inorg. Chem., 2005, 53, 268.
E. R. T. Tiekink. CrystEngComm, 2003, 5, 101. https://doi.org/10.1039/b301318a
E. R. T. Tiekink and G. Winter. Rev. Inorg. Chem., 1992, 12, 183. https://doi.org/10.1515/REVIC.1992.12.3-4.183
J. Cookson and P. D. Beer. Dalton Trans., 2007, 1459. https://doi.org/10.1039/b618088d
E. J. Mensforth, M. R. Hill, and S. R. Batten. Inorg. Chim. Acta, 2013, 403, 9. https://doi.org/10.1016/j.ica.2013.02.019
S. Nolwazi and P. A. Ajibade. J. Sulfur Chem., 2021, 42, 167-179. https://doi.org/10.1080/17415993.2020.1822838
G. Hogarth. Prog. Inorg. Chem., 2005, 53, 71.
J. D. E. T. Wilton-Ely, D. Solanki, and G. Hogarth. Eur. J. Inorg. Chem., 2005, 4027. https://doi.org/10.1002/ejic.200500430
J. D. E. T. Wilton-Ely, D. Solanki, E. R. Knight, K. B. Holt, A. L. Thompson, and G. Hogarths. Inorg. Chem., 2008, 47, 9642. https://doi.org/10.1021/ic800398b
M. J. Macgregor, G. Hogarth, A. L. Thompson, and J. D. E. T. Wilton-Ely. Organometallics, 2009, 28, 197. https://doi.org/10.1021/om800686f
V. Singh, R. Chauhan, A. N. Gupta, V. Kumar, M. G. B. Drew, L. Bahadur, and N. Singh. Dalton Trans., 2014, 43, 4752. https://doi.org/10.1039/c3dt52142g
A. Kumar, R. Chauhan, K. C. Molloy, G. Kociok-Kçhn, L. Bahadur, and N. Singh. Chem. Eur. J., 2010, 16, 4307. https://doi.org/10.1002/chem.200903367
Neetu, K. K. Manar, P. Srivastava, and N. Singh. Sol. Energy, 2018, 176, 312. https://doi.org/10.1016/j.solener.2018.10.033
G. Rajput, V. Singh, S. K. Singh, L. B. Prasad, M. G. B. Drew, and N. Singh. Eur. J. Inorg. Chem., 2012, 24, 3885. https://doi.org/10.1002/ejic.201200307
K. K. Manar, C. L. Yadav, N. Tiwari, R. K. Singh, A. Kumar, M. G. B. Drew, and N. Singh. CrystEngComm, 2017, 19, 2660. https://doi.org/10.1039/C7CE00211D
V. Kumar, V. Singh, A. N. Gupta, S. K. Singh, M. G. Drew, and N. Singh. Polyhedron, 2015, 89, 304-312. https://doi.org/10.1016/j.poly.2015.01.020
V. Kumar, V. Singh, A. N. Gupta, K. K. Manar, M. G. B. Drew, and N. Singh. CrystEngComm, 2014, 16, 6765. https://doi.org/10.1039/c4ce00510d
V. Kumar, V. Singh, A. N. Gupta, M. G. B. Drew, and N. Singh. Dalton Trans., 2015, 44, 1716. https://doi.org/10.1039/C4DT03032J
Anamika, A. K. Agrahari, K. K. Manar, C. L. Yadav, V. K. Tiwari, M. G. B. Drew, and N. Singh. New J. Chem., 2019, 43, 8939. https://doi.org/10.1039/C9NJ01551E
G. Rajput, M. K. Yadav, M. G. B. Drew, and N. Singh. Inorg. Chem., 2015, 54, 2572. https://doi.org/10.1021/ic502688h
E. R. T. Tiekink. Crystals, 2021, 11(3), 286. https://doi.org/10.3390/cryst11030286
J. Díez, M. P. Gamasa, J. Gimeno, M. Lanfranchi, and A. Triripicchio. J. Organomet. Chem., 2001, 637, 677. https://doi.org/10.1016/S0022-328X(01)00963-9
CrysAlis RED program. Abingdon, U.K.: Oxford Diffraction, 2008.
G. M. Sheldrick. Acta Crystallogr., Sect. A, 2008, 64, 112. https://doi.org/10.1107/S0108767307043930
M. N. Burnett and C. K. Johnson. ORTEP-III, Oak Ridge Thermal Ellipsoid Plot Program for Crystal Structure Illustrations, Report ORNL-6895. Oak Ridge, TN, USA: Oak Ridge National Laboratory, 1996. https://doi.org/10.2172/369685
N. Armaroli, G. Accorsi, G. Bergamini, P. Ceroni, M. Holler, O. Moudam, C. Duhayon, B. Delavaux-Nicot,and J.-F. Nierengarten. Inorg. Chim. Acta, 2007, 360, 1032. https://doi.org/10.1016/j.ica.2006.07.085
D. J. Young, S. W. Chien, and T. S. A. Hor. Dalton Trans., 2012, 41, 12655. https://doi.org/10.1039/c2dt31271a
A. Listorti, G. Accorsi, Y. Rio, N. Armaroli, O. Moudam, A. Gégout, B. Delavaux-Nicot, M. Holler,and J.-F. Nierengarten. Inorg. Chem., 2008, 47, 6254. https://doi.org/10.1021/ic800315e
T. Okubo, H. Anma, N. Tanaka, K. Himoto, S. Seki, A. Saeki, M. Maekawa, and T. Kuroda-Sowa. Chem. Commun., 2013, 49(39), 4316. https://doi.org/10.1039/C2CC37137E
E. R. T. Tiekink and J. Zukerman-Schpector. Chem. Commun., 2011, 47, 6623-6625. https://doi.org/10.1039/c1cc11173f
C. I. Yeo, S. N. A. Halim, S. W. Ng, S. L. Tan, J. Z. Schpector, M. A. B. Ferreira, and E. R. T. Tiekink. Chem. Commun., 2014, 50, 5984. https://doi.org/10.1039/C4CC02040E
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 11, pp. 1835-1843.https://doi.org/10.26902/JSC_id82655
Rights and permissions
About this article
Cite this article
Kumar, V., Singh, S. SYNTHESIS, CRYSTAL STRUCTURE, AND PROPERTIES OF HETEROLEPTIC Cu(I) DITHIOCARBAMATE COMPLEX CONTAINING DIPHENYL PHOSPHINOFERROCENE (dppf). J Struct Chem 62, 1723–1731 (2021). https://doi.org/10.1134/S0022476621110081
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621110081