Abstract
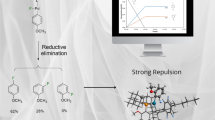
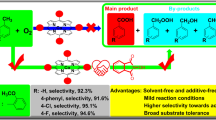
Reaction of Pd(acac)2 with BF3·OEt2 in the presence of tri(2-furyl)phosphine in toluene yields complex [Pd(acac)(TFP)2]BF4 (I) (TFP is tri(2-furyl)phosphine) whose structure is determined by XRD. The crystal structure of I contains short H⋯F contacts. The energies of these contacts are studied using DFT quantum chemical methods. Coordination complex I in combination with BF3·OEt2 in appropriate concentrations and solvents demonstrates catalytic activity in the reaction of morpholine allylic alkylation characterized by the catalyst turnover number from 198 mol to 268 mol of 4-allylmorpholine per mole of palladium and an integral selectivity to the main product of 88-99%.




Similar content being viewed by others
REFERENCES
A. I. Konovalov, I. S. Antipin, V. A. Burilov, T. I. Madzhidov, A. R. Kurbangalieva, A. V. Nemtarev, S. E. Solovieva, I. I. Stoikov, V. A. Mamedov, L. Ya. Zakharova, E. L. Gavrilova, O. G. Sinyashin, I. A. Balova, A. V. Vasilyev, I. G. Zenkevich, M. Yu. Krasavin, M. A. Kuznetsov, A. P. Molchanov, M. S. Novikov, V. A. Nikolaev, L. L. Rodina, A F. Khlebnikov, I. P. Beletskaya, S. Z. Vatsadze, S. P. Gromov, N. V. Zyk, A. T. Lebedev, D. A. Lemenovskii, V. S. Petrosyan, V. G. Nenaidenko, V. V. Negrebetskii, Yu. I. Baukov, T. A. Shmigol, A. A. Korlyukov, A. S. Tikhomirov, A. E. Shchekotikhin, V. F. Traven, L. G. Voskresenskii, F. I. Zubkov, O. A. Golubchikov, A. S. Semeikin, D. B. Berezin, P. A. Stuzhin, V. D. Filimonov, E. A. Krasnokutskaya, A. Yu. Fedorov, A. V. Nyuchev, V. Yu. Orlov, R. S. Begunov, A. I. Rusakov, A. V. Kolobov, E. R. Kofanov, O. V. Fedotova, A. Yu. Egorova, V. N. Charushin, O. N. Chupakhin, Yu. N. Klimochkin, V. A. Osyanin, A. N. Reznikov, A. S. Fisyuk, G. P. Sagitullina, A. V. Aksenov, N. A. Aksenov, M. K. Grachev, V. I. Maslennikova, M. P. Koroteev, A. K. Brel, S. V. Lisina, S. M. Medvedeva, Kh. S. Shikhaliev, G. A. Suboch, M. S. Tovbis, L. M. Mironovich, S. M. Ivanov, S. V. Kurbatov, M. E. Kletskii, O. N. Burov, K. I. Kobrakov, and D. N. Kuznetsov. Russ. J. Org. Chem., 2018, 54(2), 157-371, DOI: 10.1134/S107042801802001X.
A. S. Burlov, S. A. Mashchenko, V. G. Vlasenko, M. A. Kiskin, S. A. Nikolaevskii, and E. V. Korshunova. Russ. J. Coord. Chem., 2019, 45(11), 782-787, DOI: 10.1134/S1070328419110010.
I. A. Efimenko, A. V. Churakov, O. S. Erofeeva, N. A. Ivanova, and L. I. Demina. Russ. J. Coord. Chem., 2019, 45(9), 615-625, DOI: 10.1134/S1070328419090033.
I. A. Efimenko, M. V. Filimonova, A. V. Churakov, N. A. Ivanova, O. S. Erofeeva, A. S. Samsonova, T. S. Podosinnikova, and A. S. Filimonov. Russ. J. Coord. Chem., 2020, 46(5), 339-349, DOI: 10.1134/S1070328420040028.
E. Y. Tyulyaeva. Russ. J. Inorg. Chem., 2019, 64(14), 1775-1802, DOI: 10.1134/S0036023619140110.
V. Farina, S. R. Baker, D. A. Benigni, and C. Sapino. Tetrahedron Lett., 1988, 29 (45), 5739-5742, DOI: 10.1016/S0040-4039(00)82177-2.
V. Farina and B. Krishnan. J. Am. Chem. Soc., 1991, 113(25), 9585-9595, DOI: 10.1021/ja00025a025.
N. G. Andersen and B. A. Keay. Chem. Rev., 2001, 101(4), 997-1030, DOI: 10.1021/cr000024o.
S. Otto, A. Roodt, and J. Smith. Inorg. Chim. Acta, 2000, 303(2), 295-299, DOI: 10.1016/S0020-1693(00)00041-4.
V. Farina. Tri-2-furylphosphine. In: Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons: Chichester, UK, 2002, DOI: 10.1002/047084289X.rn00126.
M. Ackermann, A. Pascariu, T. Höcher, H. U. Siehl, and S. Berger. J. Am. Chem. Soc., 2006, 128(26), 8434-8440, DOI: 10.1021/ja057085u.
M. K. Yilmaz, S. Ince, M. Keles, and B. Güzel. J. CO2 Util., 2020, 42(August), DOI: 10.1016/j.jcou.2020.101309.
B. Yücel, L. Arve, and A. De Meijere. Tetrahedron, 2005, 61(48), 11355-11373, DOI: 10.1016/j.tet.2005.09.014.
Y. Zhang, B. Z. Lu, G.-S. Li, S. Rodriguez, J. Tan, H.-X. Wei, J. Liu, F. Roschangar, F. Ding, W. Zhao, B. Qu, D. Reeves, N. Grinberg, H. Lee, G. Heckmann, O. Niemeier, M. Brenner, Y. Tsantrizos, P. L. Beaulieu, A. Hossain, N. Yee, V. Farina, and C. H. Senanayake. Org. Lett., 2014, 16(17), 4558-4561, DOI: 10.1021/ol5021114.
D. Bouyssi, V. Gerusz, and G. Balme. Eur. J. Org. Chem., 2002, 2002(15), 2445-2448, DOI: 10.1002/1099-0690(200208)2002:15<2445::AID-EJOC2445>3.0.CO;2-S.
I. Pohorilets, M. P. Tracey, M. J. Leclaire, E. M. Moore, G. Lu, P. Liu, and K. Koide. ACS Catal., 2019, 9(12), 11720-11733, DOI: 10.1021/acscatal.9b03011.
M. Nieberding, M. P. Tracey, and K. Koide. ACS Sensors, 2017, 2(11), 1737-1743, DOI: 10.1021/acssensors.7b00697.
K. Koide, M. P. Tracey, X. Bu, J. Jo, M. J. Williams, and C. J. Welch. Nat. Commun., 2016, 7, 2-8, DOI: 10.1038/ncomms10691.
M. A. Miller, B. Askevold, H. Mikula, R. H. Kohler, D. Pirovich, and R. Weissleder. Nat. Commun., 2017, 8(May), 1-13, DOI: 10.1038/ncomms15906.
D. W. Allen, S. J. Coles, M. E. Light, and M. B. Hursthouse. Inorg. Chim. Acta, 2004, 357(5), 1558-1564, DOI: 10.1016/j.ica.2003.12.001.
S. Karmaker, S. Ghosh, S. E. Kabir, D. T. Haworth, and S. V. Lindeman. Inorg. Chim. Acta, 2012, 382(1), 199-202, DOI: 10.1016/j.ica.2011.11.053.
H. Li, C. Z. Yao, X. C. Chai, G. F. Wang, J. Li, J. Wang, Z. W. Wei, and F. L. Zhou. Mol. Cryst. Liq. Cryst., 2018, 664 (1), 156-164, DOI: 10.1080/15421406.2018.1461837.
R. Meijboom and A. Muller. Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62(10), m2642-m2644, DOI: 10.1107/S1600536806036634.
F. Wu, H. Wang, and W. Chen. Appl. Organomet. Chem., 2019, 33(3), e4775, DOI: 10.1002/aoc.4775.
D. E. Jenkins, R. E. Sykora, and Z. Assefa. Inorg. Chim. Acta, 2013, 406, 293-300, DOI: 10.1016/j.ica.2013.04.047.
F. Bachechi, A. Burini, R. Galassi, and B. R. Pietroni. J. Mol. Struct., 2005, 740(1-3), 119-123, DOI: 10.1016/j.molstruc.2005.01.030.
C. M. Hettrick and W. J. Scott. J. Am. Chem. Soc., 1991, 113(13), 4903-4910, DOI: 10.1021/ja00013a028.
D. S. Suslov, M. V. Pakhomova, M. V. Bykov, I. A. Ushakov, and V. S. Tkach. Catal. Commun., 2019, 119, 16-21, DOI: 10.1016/j.catcom.2018.10.010.
D. S. Suslov, M. V. Bykov, A. V. Kuzmin, P. A. Abramov, O. V. Kravchenko, M. V. Pakhomova, A. V. Rokhin, I. A. Ushakov, and V. S. Tkach. Catal. Commun., 2018, 106, 30-35, DOI: 10.1016/j.catcom.2017.12.010.
D. S. Suslov, M. V. Bykov, M. V. Pakhomova, P. A. Abramov, I. A. Ushakov, and V. S. Tkach. Catal. Commun., 2017, 94, 69-72, DOI: 10.1016/j.catcom.2017.02.004.
D. S. Suslov, M. V. Bykov, Z. D. Abramov, I. A. Ushakov, T. N. Borodina, V. I. Smirnov, G. V. Ratovskii, and V. S. Tkach. J. Organomet. Chem., 2020, 923, 121413, DOI: 10.1016/j.jorganchem.2020.121413.
G. M. Sheldrick. Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64(1), 112-122, DOI: 10.1107/S0108767307043930.
G. M. Sheldrick. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71(1), 3-8, DOI: 10.1107/S2053229614024218.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42(2), 339-341, DOI: 10.1107/S0021889808042726.
F. Neese, F. Wennmohs, U. Becker, and C. Riplinger. J. Chem. Phys., 2020, 152(22), 224108, DOI: 10.1063/5.0004608.
F. Neese. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2(1), 73-78, DOI: 10.1002/wcms.81.
A. D. Becke. Phys. Rev. A, 1988, 38, 3098-3100.
J. P. Perdew. Phys. Rev. B, 1986, 33, 8822-8824.
K. Eichkorn, O. Treutler, H. Öhm, M. Häser, and R. Ahlrichs. Chem. Phys. Lett., 1995, 242, 652-660.
F. Weigend. Phys. Chem. Chem. Phys., 2006, 8(9), 1057-1065, DOI: 10.1039/b515623h.
F. Weigend and R. Ahlrichs. Phys. Chem. Chem. Phys., 2005, 7(18), 3297-3305, DOI: 10.1039/b508541a.
D. Andrae, U. Haussermann, M. Dolg, H. Stoll, and H. Preuss. Theor. Chim. Acta, 1990, 77(2), 123-141, DOI: 10.1007/BF01114537.
M. Bühl and H. Kabrede. J. Chem. Theory Comput., 2006, 2, 1282-1290, DOI: 10.1021/ct6001187.
M. Bühl, C. Reimann, D. A. Pantazis, T. Bredow, and F. Neese. J. Chem. Theory Comput., 2008, 4(9), 1449-1459, DOI: 10.1021/ct800172j.
C. J. Cramer and D. G. Truhlar. Phys. Chem. Chem. Phys., 2009, 11(46), 10757-10816, DOI: 10.1039/b907148b.
M. Waller, H. Braun, N. Hojdis, and M. Bühl. J. Chem. Theory Comput., 2007, 3, 2234-2242.
M. J. Szabo, R. F. Jordan, A. Michalak, W. E. Piers, T. Weiss, S.-Y. Yang, and T. Ziegler. Organometallics, 2004, 23(23), 5565-5572, DOI: 10.1021/om049485g.
S. Tobisch and T. Ziegler. J. Am. Chem. Soc., 2004, 126(29), 9059-9071, DOI: 10.1021/ja048861l.
R. F. W. Bader. Chem. Rev., 1991, 91(5), 893-928, DOI: 10.1021/cr00005a013.
T. Lu and F. Chen. J. Comput. Chem., 2012, 33(5), 580-592, DOI: 10.1002/jcc.22885.
V. S. Tkach, D. S. Suslov, G. Myagmarsuren, G. V. Ratovskii, A. V. Rohin, F. Tuczek, and F. K. Shmidt. J. Organomet. Chem., 2008, 693(12), 2069-2073, DOI: 10.1016/j.jorganchem.2007.12.019.
D. S. Suslov, M. V. Bykov, M. V. Pakhomova, Z. D. Abramov, G. V. Ratovskii, I. A. Ushakov, T. N. Borodina, V. I. Smirnov, and V. S. Tkach. J. Mol. Struct., 2020, 1217, 128425, DOI: 10.1016/j.molstruc.2020.128425.
J. Vicente, A. Arcas, D. Bautista, A. Tiripicchio, and M. Tiripicchio-Camellini. New J. Chem., 1996, 20, 345-356.
V. S. Tkach, D. S. Suslov, N. V. Kurateva, M. V. Bykov, and M. V. Belova. Russ. J. Coord. Chem., 2011, 37(10), 752-756, DOI: 10.1134/S1070328411090119.
N. V. Kuratieva, V. S. Tkach, D. S. Suslov, M. V. Bykov, and S. A. Gromilov. J. Struct. Chem., 2011, 52(4), 813-855, DOI: 10.1134/S0022476611040263.
A. S. Novikov, D. M. Ivanov, M. S. Avdontceva, and V. Y. Kukushkin. CrystEngComm, 2017, 19(18), 2517-2525, DOI: 10.1039/C7CE00346C.
S. A. Adonin, A. S. Novikov, and V. P. Fedin. Russ. J. Coord. Chem., 2020, 46(1), 37-41, DOI: 10.1134/S1070328420010017.
A. S. Novikov. Crystals, 2020, 10(6), 537, DOI: 10.3390/cryst10060537.
E. Espinosa, I. Alkorta, J. Elguero, and E. Molins. J. Chem. Phys., 2002, 117(12), 5529-5542, DOI: 10.1063/1.1501133.
Q. He, J. Yang, and X. Meng. Chin. J. Chem. Phys., 2009, 22(5), 517-522, DOI: 10.1088/1674-0068/22/05/517-522.
E. V. Bartashevich and V. G. Tsirelson. Russ. Chem. Rev., 2014, 83(12), 1181-1203, DOI: 10.1070/RCR4440.
B. M. Trost and M. L. Crawley. Chem. Rev., 2003, 103(8), 2921-2944, DOI: 10.1021/cr020027w.
T. Graening and H.-G. Schmalz. Angew. Chem., 2003, 115(23), 2684-2688, DOI: 10.1002/ange.200301644.
B. M. Trost and M. L. Crawley. In: Transition Met. Catal. Enantiosel. Allylic Substitution Org. Synth. / Ed. U. Kazmaier, Springer-Verlag: Berlin, Heidelberg, 2011, 321-340.
B. M. Trost and T. J. Fullerton. J. Am. Chem. Soc., 1973, 95(1), 292-294, DOI: 10.1021/ja00782a080.
I. Shimizu, T. Yamada, and J. Tsuji. Tetrahedron Lett., 1980, 21(33), 3199-3202, DOI: 10.1016/S0040-4039(00)77444-2.
A. A. Vasilev, I. M. Aladzheva, and O. V. Bykhovskaya. Russ. Chem. Bull., 2017, 66(4), 661-665, DOI: 10.1007/s11172-017-1788-6.
A. A. Vasilev, S. E. Lyubimov, E. P. Serebryakov, V. A. Davankov, M. I. Struchkova, and S. G. Zlotin. Russ. Chem. Bull., 2010, 59(3), 605-610, DOI: 10.1007/s11172-010-0120-5.
H. Kinoshita, H. Shinokubo, and K. Oshima. Org. Lett., 2004, 6(22), 4085-4088, DOI: 10.1021/ol048207a.
Y. Gumrukcu, B. de Bruin, and J. N. H. Reek. ChemSusChem, 2014, 7(3), 890-896, DOI: 10.1002/cssc.201300723.
M. Kimura, M. Fukasaka, and Y. Tamaru. Heterocycles, 2006, 67(2), 535-542, DOI: 10.3987/COM-05-S(T)63.
X. Huo, G. Yang, D. Liu, Y. Liu, I. D. Gridnev, and W. Zhang. Angew. Chem., Int. Ed., 2014, 53(26), 6776-6780, DOI: 10.1002/anie.201403410.
D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer Jr., R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zakshand, and T. Y. Zhang. Green Chem., 2007, 9(5), 411-420, DOI: 10.1039/B703488C.
E. Espinosa, E. Molins, and C. Lecomte. Chem. Phys. Lett., 1998, 285(3-4), 170-173, DOI: 10.1016/S0009-2614(98)00036-0.
M. V. Vener, A. N. Egorova, A. V. Churakov, and V. G. Tsirelson. J. Comput. Chem., 2012, 33(29), 2303-2309, DOI: 10.1002/jcc.23062.
M. Kimura, M. Futamata, K. Shibata, and Y. Tamaru. Chem. Commun., 2003, 2003(2), 234-235, DOI: 10.1039/b210920d.
M. Kimura and Y. Tamaru. Mini-Rev. Org. Chem., 2009, 6(4), 392-397.
Funding
The study was supported by the Russian Science Foundation (project No. 19-73-00046).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 8, pp. 1305-1316.https://doi.org/10.26902/JSC_id81367
Rights and permissions
About this article
Cite this article
Bykov, M.V., Abramov, Z.D., Orlov, T.S. et al. STRUCTURE AND CATALYTIC PROPERTIES OF (ACETYLACETONATO-κ2O,O′)BIS(TRI(2-FURYL)PHOSPHINE) PALLADIUM(II) TETRAFLUOROBORATE. J Struct Chem 62, 1218–1228 (2021). https://doi.org/10.1134/S0022476621080072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621080072