Abstract
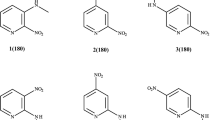
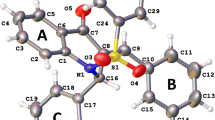
Crystal structures of three isomeric styryldiazine derivatives (E)-2-(3,4-dimetoxystryl)pyrazine, (E)-2-(3,4-dimetoxystryl)pyrimidine, and (E)-4-(3,4-dimetoxystryl)pyrimidine with different positions of nitrogen atoms are studied. These compounds can be used as potential initial compounds for biologically active (antibacterial or antitumor) agents, pesticides, fungicides, dyes, analytical reagents. Even though these molecules have similar chemical structures and geometric parameters in the crystal, the crystal packing motifs of these three compounds differ significantly. The conducted PBE0/6-31G(d,p) quantum chemical calculations show that these molecules may have several conformers with close energies, including those observed in the crystal, and that these forms may transform into each other through barriers not exceeding 43.5 kJ/mol. The highest density at 120 K and the highest energy of crystal lattice calculated by the UNI force field are observed for the pyrimidine derivative (E)-2-(3,4-dimetoxystryl)pyrimidine (6). This is the only structure that exhibits photocyclization in solid state due to the fact that its crystal contains centrosymmetric dimers with a distance between the atoms of olefin fragments equal to ≈ 3.9 Å.







Similar content being viewed by others
REFERENCES
V. Botti, F. Elisei, U. Mazzucato, I. Šagud, M. Šindler-Kulykand, and A. Spalletti. J. Photochem. Photobiol. Chem., 2016, 316, 95-103.
O. A. Fedorova, E. N. Gulakova, Y. V. Fedorov, I. E. Lobazova, M. V. Alfimov, and G. Jonusauskas. J. Photochem. Photobiol. Chem., 2008, 196, 239-245.
S. Yamada, K. Yamagami, and S. Oaku. Tetrahedron Lett., 2016, 57, 2451-2454.
A. Misale, S. Niyomchon, and N. Maulide. Acc. Chem. Res., 2016, 49, 2444-2458.
W. A. Smit, A. F. Bochkov, and R. Caple. Organic Synthesis: The Science behind the Art. The Royal Society of Chemistry, 1998.
R. G. Mortimer. Mathematics for Physical Chemistry, 4th ed. Academic Press: Amsterdam, 2013.
E. N. Ushakov and S. P. Gromov. Russ. Chem. Rev., 2015, 84, 787-802.
D. V. Berdnikova, T. M. Aliyeu, S. Delbaere, Y. V. Fedorov, G. Jonusauskas, V. V. Novikov, A. A. Pavlov, A. S. Peregudov, N. E. Shepel′, F. I. Zubkov, and O. A. Fedorova. Dyes Pigm., 2017, 139, 397-402.
O. A. Fedorova, A. E. Saifutiarova, E. N. Gulakova, E. O. Guskova, T. M. Aliyeu, N. E. Shepel, and Y. V. Fedorov. Photochem. Photobiol. Sci., 2019, 18, 2208-2215.
A. V. Vologzhanina. Crystals, 2019, 9, 478.
E. N. Gulakova, D. V. Berdnikova, T. M. Aliyeu, Y. V. Fedorov, I. A. Godovikov, and O. A. Fedorova. J. Org. Chem., 2014, 79, 5533-5537.
SAINT v8.34A. Bruker AXS Inc.: Madison, Wisconsin, USA, 2013.
L. Krause, R. Herbst-Irmer, G. M. Sheldrick, and D. Stalke. J. Appl. Crystallogr., 2015, 48, 3-10.
G. M. Sheldrick. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3-8.
G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3-8.
C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek, and P. A. Wood. J. Appl. Crystallogr., 2008, 41, 466-470.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox. Gaussian 09, Wallingford, CT: Gaussian, 2009.
C. Adamo and V. Barone. J. Chem. Phys., 1999, 110, 6158.
T. A. Keith. AIMAll (Version 19.10.12). TK Gristmill Software: Overland Park, KS, USA, 2019.
T. M. Aliyeu, D. V. Berdnikova, O. A. Fedorova, E. N. Gulakova, C. Stremmel, and . Ihmels. J. Org. Chem., 2016, 81, 9075-9085.
I. J. Bruno, J. C. Cole, M. Kessler, J. Luo, W. D. S. Motherwell, L. H. Purkis, B. R. Smith, R. Taylor, R. I. Cooper, S. E. Harris, and A. G. Orpen. J. Chem. Inf. Comput. Sci., 2004, 44, 2133-2144.
P. C. Chen and Y. C. Chieh. J. Mol. Struct.: THEOCHEM, 2003, 624, 191-200.
Y. V. Nelyubina, I. V. Glukhov, M. Yu. Antipin, and K. A. Lyssenko. Chem. Commun., 2010, 46, 3469.
I. L. Dalinger, A. V. Kormanov, K. Yu. Suponitsky, N. V. Muravyev, and A. B. Sheremetev. Chem. - Asian J., 2018, 13, 1165-1172.
A. Gavezzotti. Acc. Chem. Res., 1994, 27, 309-314.
A. Bondi. J. Phys. Chem., 1964, 68, 441-451.
A. A. Gidaspov, V. A. Zalomlenkov, V. V. Bakharev, V. E. Parfenov, E. V. Yurtaev, M. I. Struchkova, N. V. Palysaeva, K. Y. Suponitsky, D. B. Lempert, and A. B. Sheremetev. RSC Adv., 2016, 6, 34921-34934.
A. B. Sheremetev, N. S. Aleksandrova, S. S. Semyakin, K. Yu. Suponitsky, and D. B. Lempert. Chem. - Asian J., 2019, 14, 4255-4261.
A. V. Maleev, A. A. Gevorgyan, and K. A. Potekhin. J. Struct. Chem., 2018, 59(2), 455-462.
Funding
The reported study was funded by RFBR, project number 19-33-90227. The structures were determined with the support of the Ministry of Science and Higher Education of the Russian Federation using the equipment of the Center for the Study of Molecular Structures INEOS RAS. The quantum chemical calculations were performed using resources of the Siberian Supercomputing Centre of ICMMG SB RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 4, pp. 565-574.https://doi.org/10.26902/JSC_id71040
Rights and permissions
About this article
Cite this article
Saifutiarova, A.E., Karnoukhova, V.A., Gulakova, E.N. et al. MOLECULAR STRUCTURES AND CRYSTAL PACKINGS OF STYRYLDIAZINES. J Struct Chem 62, 527–536 (2021). https://doi.org/10.1134/S002247662104003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002247662104003X