Abstract
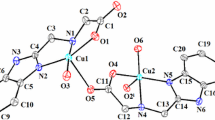
Monomeric [Cu(DCA–OEt)2] and polymeric [Cu(DCA)2(DMF)2]n complexes (where DCA is dicyanamide, DCA–OEt is in situ formed ligand, and DMF is dimetylformamide) derived from sodium dicyanamide are synthesized and their structures are characterised by spectroscopic and analytical methods. Molecular structures of the complexes are determined by single crystal X-ray diffraction studies. In the structure of the polymeric complex [Cu(DCA)2(DMF)2]n, the DCA anions are bridges between Cu(II) ions through the terminal nitrile N atoms, forming a 1D ladder-like coordination polymer chains. The DCA–OEt ligand in the monomeric [Cu(DCA–OEt)2] complex is formed by the nucleophilic addition of ethanol to the DCA nitrile groups, resulting in a bidentate chelate ligand. The antibacterial activity of the structurally characterised complexes are evaluated by by agar-well diffusion. The complexes exhibit a comparable antibacterial activity against some bacteria to the standart antibiotics (amikacin and gentamicin).






Similar content being viewed by others
REFERENCES
M. Kurmoo and C. J. Kepert. New J. Chem., 1998, 22(12), 1515.
L. Tabrizi, H. Chiniforoshan, J. P. Araújo, A. M. Lopes, H. Görls, W. Plass, and G. Mohammadnezhad. Inorg. Chim. Acta, 2015, 426, 195.
S .R. Batten, R. Robson, P. Jensen, B. Moubaraki, and K. S. Murray. Chem. Commun., 1998, 439.
L. L. Zheng. J. Inorg. Chem., 2013, 2013, 1.
L. E. Blidi, J. Saleh, O. B. Ghanem, M. El-Harbawi, J. M. Lévêque, and M. K. Hadj-Kali. J. Chem. Eng. Data, 2020, 65(1), 34.
F. N. Shirota, E. G. Demaster, and H. T. Nagasawa. Biochem. Pharm., 1982, 31(11), 1999.
F. Saczewski and L. Balewski. Expert Opin. Ther. Pat., 2009, 19(10), 1417.
B. H. Mashat. Br. J. Environ. Sci., 2016, 4(1), 49.
Bruker APEX2 and SAINT. Bruker AXS Inc, 1998.
G. M. Sheldrick. Acta Crystallogr., Sect. A, 2008, 64, 112.
G. M. Sheldrick. Acta Crystallogr., Sect. C, 2015, 71, 3.
M. Balouiri, M. Sadiki, and S. K. Ibnsouda. J. Pharm. Anal., 2016, 6(2), 71.
A. W. Bauer, W. M. M. Kirby, J. C. Sherris, and M. Turck. Am. J. Clin. Pathol., 1966, 45, 493.
P. R. Murray, E. Baron, M. A. Pfaller, F. C. Tenover, H. R. Yolke. In: Manual of Clinical Microbiology / Ed. Patrick R. Murray. ASM Press: Washington, D.C., 1995, 2488.
A. P. David and J. P. McCuen. In: Manual of BBL Products and Laboratory Procedures / Eds D. A. Power and P. J. McCuen. Beckton Dickinson and Co.: Cockeysville, MD, 1988, 67.
CLSI, Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard, 7th ed., CLSI document M02-A11. Clinical and Laboratory Standards Institute: Wayne, PA, 2012.
S. B. Bayar, Z. Hocaoğlu, and M. Dığrak. KSU J. Nat. Sci., 2008, 11(1), 1.
L. L. Zheng, J. D. Leng, W. T. Liu, W. X. Zhang, J. X. Lu, and M. L. Tong. Eur. J. Inorg. Chem., 2008, 2008(29), 4616.
M. M. Bishop, L. F. Lindoy, S. Mahadev, and P. Turner. J. Chem. Soc., Dalton Trans., 2000, 233.
I. M. Atkinson, M. M. Bishop, L. F. Lindoy, S. Mahadev, and P. Turner. Chem. Commun., 2002, 23, 2818.
M. Kose, C. Hepokur, D. Karakas, V. McKee, and M. Kurtoglu. Polyhedron, 2016, 117, 652.
M. Kose, S. E. Duman, V. McKee, I. Akyol, and M. Kurtoglu. Inorg. Chim. Acta, 2017, 462, 281.
R. Olar, M. Badea, D. Marinescu, C. M. Chifiriuc, C. Bleotu, M. N. Grecu, E. E. Iorgulescu, M. Bucur, V. Lazar, and A. Finaru. Eur. J. Med. Chem., 2010, 45, 2868.
F. Bentefrit, G. Morgant, B. Viossat, S. Leonce, N. Guilbaud, A. Pierre, G. Atassi, and H. D. Nguyen. J. Inorg. Biochem., 1997, 68(1), 53.
S. P. Devi, R. K. B. Devi, N. S. Devi, L. J. Singh, and R. K. H. Singh. Polyhedron, 2012, 47(1), 1.
E. G. Demaster, B. Redfern, and H. T. Nagasawa. Biochem. Pharmacol., 1998, 55(12), 2007.
S. N. Kertmen, I. Gonul, and M. Kose. J. Mol. Struct., 2018, 1152, 29.
S. V. K. Nune, A. T. Basaran, E. Ülker, R. Mishra, and F. Karadas. ChemCatChem, 2017, 9(2), 300.
J. L. Manson, J. A. Schlueter, and C. L. Nygren. Dalton Trans., 2007, 6, 646.
A. C. B. Yuoh, M. O. Agwara, D. M. Yufanyi, M. A. Conde, R. Jagan, and K. O. Eyong. Int. J. Inorg. Chem., 2015, 2015, 1.
T. Chattopadhyay, A. Banerjee, K. S. Banu, N. Podder, M. Mukherjee, M. Ghosh, and D. Das. J. Mol. Struct., 2008, 888, 62.
D. S. Tonzing, S. R. Batten, and K. S. Murray. J. Mol. Struct., 2006, 796, 63.
P. P. J. Figiel, M. N. Kopylovich, J. Lasri, M. F. C. G da Silva, J. J. F. da Silva, and A. J. Pombeiro. Chem. Commun., 2010, 46(16), 2766.
S. Jana, S. Khan, A. Bauzá, A. Frontera, and S. Chattopadhyay. J. Mol. Struct., 2017,
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Kurtar, S.N.K., Koçer, F. & Kose, M. MONOMERIC AND 1D POLYMERIC Cu(II) COMPLEXES DERIVED FROM DICYANAMIDE: STRUCTURAL CHARACTERIZATION AND ANTIBACTERIAL PROPERTIES. J Struct Chem 61, 1296–1305 (2020). https://doi.org/10.1134/S0022476620080168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476620080168