Abstract
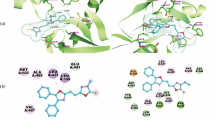
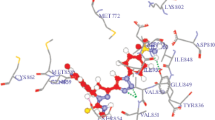
The 1,2,3,4-tetrahydroisoquinoline derivative compound fused with thiophene (BDTTIQ) is synthesized by the slow evaporation solution growth method and characterized by SCXRD, 1H and 13C NMR techniques. In the synthesized compound, the tetrahydroisoquinoline fragment of BDTTIQ is almost in the half-chair conformation. A 3D supramolecular architecture is attained by intermolecular C-H...0 and C-H...71 interactions in the crystal structure. Molecular docking simulations are carried out to examine the inhibitory nature of the synthesized compound BDTTIQ against ARK1C3 protein.
Similar content being viewed by others
References
K. W. Bentley. In: The Isoquinoline Alkaloids. Harwood Acad. Publ. Amsterdam, 1998.
J. D. Scott and R. M. Williams. Chem. Rev., 2002, 102(5), 1669–730.
P. Siengalewicz, U. Rinner, and J. Mulzer. Chem. Soc. Rev., 2008, 37(12), 2676–90.
M. Yepuru, Z. Wu, A. Kulkarni, F. Yin, C.M. Barrett, J. Kim, M. S. Steiner, D. D. Miller, J. T. Dalton, and R. Narayanan. Clin. Cancer Res., 2013, 19(20), 5613–5625.
A. O. Adeniji, M. Chen, and T. M. Penning, J. Steroid Biochem. Mol. Biology, 2013, 137, 136–49.
Doxorubicin. Martindale: The Complete Drug Reference / Ed. A. Brayfield. Pharmaceutical Press, 2014.
Australian Medicines Hand book Adelaide / Ed. S. Rossi. The Australian, Medicines Hand book unit trust, 2013.
R. Pingaew, A. Saekee, P. Mandi, C. Nantasenamat, S. Prachayasittikul, S. Ruchirawat, and V. Prachayasittikul. Eur. J. Med. Chem., 2014, 85, 65–76.
X. Zheng, Y. Wu, D. Wu, X. Wang, C. Zhang, X. Guo, and H. B. Luo. Bioorgan. Med. Chem. Lett., 2016, 26(23), 5631–5638.
(a) Bruker AP, SAINT A. Inc., Madison, WI, 2004, Search PubMed; (b) G. M. Sheldrick. Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112.
G. M. Sheldrick. Acta. Cryst., 2008, 64A, 112–122.
G. M. Sheldrick. SHELXS97, A Program for Crystal Structure Solution. University of Gottingen: Germany, 1997.
L. J. Farrugia. J. Appl. Crystallogr., 2012, 45(4), 849–54.
G. M. Sheldrick. SHELXL97, A Program for Crystal Structure Refinement. University of Gottingen: Germany, 1997.
A. L. Spek. J. Appl. Crystallogr., 2003, 36(1), 7–13.
L. J. Farrugia. J. Appl. Crystallogr., 1997, 30(5/1), 565.
I. J. Bruno, J. C. Cole, P. R. Edgington, M. Kessler, C. F. Macrae, P. McCabe, J. Pearson, and R. Taylor. Acta Crystallogr. Sec. B, 2002, 55(3/1), 389–97.
Discovery Studio 2015, Dassault systems BIOVIA, Discovery Studio Modeling Enviroment, Release 4.5, DassaultSytems: SanDiego.
W. L. Duax, C. M. Weeks, and D. C. Rohrer. Topics in Stereochemistry, Vol. 9 / Ed. E. L. Eliel, N. Allinger.
D. Cremer, and J. A. Pople. J. Am. Chem. Soc, 1975, 97, 1354.
S. Murugavel, N. Manikandan, D. Lakshmanan, K. Naveen, and P. T. Perumal. J. Chilean Chem. Soc, 2015, 60(3), 3015–20.
Acknowledgements
The authors thank Dr. Babu Vargheese, SAIF, IIT, Madras, India, for his help with the data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Text © The Author(s), 2019, published in Zhurnal Strukturnoi Khimii, 2019, Vol. 60, No. 7, pp. 1190-1196.
Electronic supplementary material
10947_2019_1233_MOESM1_ESM.pdf
Supplementary Materials to: Synthesis, Structural Commentary, Supramolecular Architecture and Molecular Docking Investigations of a Novel Thiophene-Fused 1,2,3,4-Tetrahydroisoquinoline Derivative as a Potent Anti-Cancer Agent
Rights and permissions
About this article
Cite this article
Murugavel, S., Manikandan, N., Ravikumar, C. et al. Synthesis, Structural Commentary, Supramolecular Architecture and Molecular Docking Investigations of a Novel Thiophene-Fused 1,2,3,4-Tetrahydroisoquinoline Derivative as a Potent Anti-Cancer Agent. J Struct Chem 60, 1143–1149 (2019). https://doi.org/10.1134/S0022476619070163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476619070163