Abstract
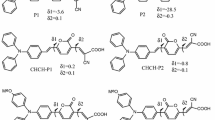
Titanium dioxide nanoparticles representing those in dye-sensitized solar cell photoanodes are modeled by first principles calculations, employing a series of structurally resolved polyoxometalates functionalized with organic ligands via the phosphonate anchoring group as a modeling platform. Previous computational studies on titanium dioxide nanoparticles for dye-sensitized solar cells and water splitting systems are based on artificial cleaving of TiO2 from bulk crystals, which introduces potential various human-made errors. This manuscript focuses on structurally resolved titanium dioxide nanoparticles determined from X-ray diffraction experiments with a 10−3 Å resolution and demonstrates that charge transfer occurs from the organic ligands and oxygen atoms to the core titanium atoms. Also, different TiO2 nanoparticle geometries contribute to variation in the electronic and optical properties of the organic/TiO2 nanocomposite system. This computational work on structurally resolved molecularly functionalized titanium dioxide introduces a new way to model the TiO2 nanoparticle-based optoelectronic device, which eliminates the arbitrariness introduced during artificial cleaving and provides insights on the structure-property relationships of organic molecule-functionalized titanium dioxide nanoparticles for water splitting systems and dye-sensitized solar cells.
Similar content being viewed by others
References
B. O’Regan, and M. Grätzel. Nature, 1991, 353, 737–740. doi: https://doi.org/10.1038/353737a0
J. R. Swierk, D. D. Méndez-Hernández, N. S. McCool et al. Proc. Natl. Acad. Sci., USA. 2015, 112, 1681–1686. doi: https://doi.org/10.1073/pnas.1414901112
J. R. Swierk and T. E. Mallouk. Chem. Soc. Rev., 2013, 42, 2357–2387. doi: https://doi.org/10.1039/C2CS35246J
S. Ardo and G. J. Meyer. Chem. Soc. Rev., 2009, 38, 115–164. doi: https://doi.org/10.1039/b804321n
M. Grätzel. Nature, 2001, 414, 338–44. doi: https://doi.org/10.1038/35104607
L. Sang, Y. Zhao, and C. Burda. Chem. Rev., 2014, 114, 9283–9318. doi: https://doi.org/10.1021/cr400629p
J. Rochford, D. Chu, A. Hagfeldt, and E. Galoppini. J. Am. Chem. Soc., 2007, 129, 4655–4665. doi: https://doi.org/10.1021/ja068218u
M. Ferus, L. Kavan, M. Zukalová et al. J. Phys. Chem. C, 2014, 118, 26845–26850. doi: https://doi.org/10.1021/jp5090668
S. Civiš, M. Ferus, M. Zukalová et al. J. Phys. Chem. C, 2015, 119, 3605–3612. doi: https://doi.org/10.1021/jp512059b
F. Labat, T. Le Bahers, I. Ciofini, and C. Adamo. Acc. Chem. Res., 2012, 45, 1268–1277. doi: https://doi.org/10.1021/ar200327w
M. Pastore and and F. De Angelis. J. Am. Chem. Soc., 2015, 137, 5798–5809. doi: https://doi.org/10.1021/jacs.5b02128
F. De Angelis, C. Di Valentin, S. Fantacci et al. Chem. Rev., 2014, 114, 9708–9753. doi: https://doi.org/10.1021/cr500055q
M. D. Kärkäs, O. Verho, E. V. Johnston, and B. Åkermark. Chem. Rev., 2014, 114, 11863–2001. doi: https://doi.org/10.1021/cr400572f
L. Zhang, J. M. Cole, and C. Dai. ACS Appl. Mater. Interfaces, 2014, 6, 7535–7546. doi: https://doi.org/10.1021/am502186k
E. Ronca, M. Pastore, L. Belpassi et al. Energy Environ. Sci., 2013, 6, 183. doi: https://doi.org/10.1039/c2ee23170k
F. Ambrosio, N. Martsinovich, and A. Troisi. J. Phys. Chem. Lett., 2012, 3, 1531–1535. doi: https://doi.org/10.1021/jz300520p
S. Zhang, W. Zhou, Y. Ma et al. Nano Lett., 2017, 17, 3434–3440. doi: https://doi.org/10.1021/acs.nanolett.7b00297
S. Zhang, Z. Yan, Y. Li et al. Angew. Chemie, 2015, 127, 3155–3158. doi: https://doi.org/10.1002/ange.201411246
J. B. Benedict and P. Coppens. J. Am. Chem. Soc., 2010, 132, 2938–2944. doi: https://doi.org/10.1021/ja909600w
P. Coppens, Y. Chen, and E. Trzop. Chem. Rev., 2014, 114, 9645–9661. doi: https://doi.org/10.1021/cr400724e
J. B. Benedict, R. Freindorf, E. Trzop et al. J. Am. Chem. Soc., 2010, 132, 13669–71. doi: https://doi.org/10.1021/ja106436y
R. C. Snoeberger, K. J. Young, J. Tang et al. J. Am. Chem. Soc., 2012, 134, 8911–8917. doi: https://doi.org/10.1021/ja301238t
J. D. Sokolow, E. Trzop, Y. Chen et al. J. Am. Chem. Soc., 2012, 134, 11695–11700. doi: https://doi.org/10.1021/ja303692r
M. W. Kryman, J. N. Nasca, D. F. Watson, and M. R. Detty. Langmuir, 2016, 32, 1521–1532. doi: https://doi.org/10.1021/acs.langmuir.5b04275
W. M. Campbell, A. K. Burrell, D. L. Officer, and K. W. Jolley. Coord. Chem. Rev., 2004, 248, 1363–1379. doi: https://doi.org/10.1016/j.ccr.2004.01.007
J. Cui, J. Lu, X. Xu et al. J. Phys. Chem. C, 2014, 118, 16433–16440. doi: https://doi.org/10.1021/jp410829c
Y. Chen, E. Trzop, J. D. Sokolow, and P. Coppens. Chem. — A Eur. J., 2013, 19, 16651–16655. doi: https://doi.org/10.1002/chem.201302012
K. N. Jarzembska, Y. Chen, J. N. Nasca et al. Phys. Chem. Chem. Phys., 2014, 16, 15792. doi: https://doi.org/10.1039/C4CP02509A
B. Delley. J. Chem. Phys., 2000, 113, 7756–7764. doi: https://doi.org/10.1063/1.1316015
A. Tkatchenko and M. Scheffler. Phys. Rev. Lett., 2000, 102, 073005. doi: https://doi.org/10.1103/PhysRevLett.102.073005
F. Nunzi, E. Mosconi, L. Storchi et al. Energy Environ. Sci., 2013, 6, 1221. doi: https://doi.org/10.1039/c3ee24100a
S. K. Wallace and K. P. Mckenna. Adv. Mater. Interfaces, 2014, 1, 1400078. doi: https://doi.org/10.1002/admi.201400078
Y. Chen, K. N. Jarzembska, E. Trzop et al. Chem. a Eur. J., 2015, 21, 11538–11544. doi: https://doi.org/10.1002/chem.201500961
L. Zhang and J. M. Cole. ACS Appl. Mater. Interfaces, 2014, 6, 15760–15766. doi: https://doi.org/10.1021/am502687k
L. Zhang, J. M. Cole, P.G. Waddell et al. ACS Sustain Chem. Eng., 2013, 1, 1440–1452. doi: https://doi.org/10.1021/sc400183t
M. Planells, L. Pellejà, J. N. Clifford et al. Energy Environ. Sci., 2011, 4, 1820. doi: https://doi.org/10.1039/c1ee01060c
C. F. A. Negre, K. J. Young, M. B. Oviedo et al. J. Am. Chem. Soc., 2014, 136, 16420–9. doi: https://doi.org/10.1021/ja509270f
J. D. Sokolow, E. Trzop, Y. Chen et al. J. Am. Chem. Soc., 2012, 134, 11695–11700. doi: https://doi.org/10.1021/ja303692r
Author information
Authors and Affiliations
Corresponding author
Additional information
Text © The Author(s), 2019, published in Zhurnal Strukturnoi Khimii, 2019, Vol. 60, No. 4, pp. 697–703.
Electronic Supplementary Material
10947_2019_1182_MOESM1_ESM.pdf
Supplementary Materials to: First Principles Study on Structurally Resolved Titanium Dioxide Nanoparticles Functionalized by Organic Ligands
Rights and permissions
About this article
Cite this article
Zhang, L., Xu, L. & Li, J. First Principles Study on Structurally Resolved Titanium Dioxide Nanoparticles Functionalized by Organic Ligands. J Struct Chem 60, 671–677 (2019). https://doi.org/10.1134/S002247661904019X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002247661904019X