Abstract
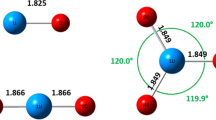
Theoretical studies on the lanthanide and actinide triflate complexes M(OTf) n where M = La, Ce, Gd, Yb, Lu, Th, U, Np, Pu, Am, Cm, Bk, and No; n = 3 and 4, are carried out using functional density theory (DFT). The study of An(OTf)3 complexes showed that the three OTf groups are bidentate, generating a trigonal prism (TP). Two limiting structures of TP are observed; the most distorted is the thorium triflate Th(OTf)3 and the ideal one is U(OTf)3. The highest population contribution of 5d orbital compared to 5f orbital in Th–O bond of Th(OTf)3 explains the distortion. The intramolecular rearrangement of the OTf ligands in Ln(OTf)3 generates two conformers. In Yb(OTf)3, the pseudo-eclipsed and the staggered conformations are stable and can be isolated.
Similar content being viewed by others
References
C. Piguet and J. C. G. Bünzli, Chem. Soc. Rev., 28, 347–358 (1999).
K. A. Gschneidner, L. Eyring, G. R. Chopin, and G. H. Lander (eds.), Handbook on the Physics and Chemistry of Rare Earths. Lanthanides/Actinides: Chemistry, Elsevier Science (1994), p. 18.
a) S. Kobayashi, Synlett, 689 (1994)
F. T. Edelmann, New J. Chem., 19, 535 (1995)
H. Schumann, J. A. Meese-Marktscheffel, A. Dietrich, and F. H. Görlitz, J. Organomet. Chem., 299, 430 (1992).
G. A. Lawrance, Chem. Rev., 17, 86 (1986).
Actinides and Fission Products Partitioning and Transmutation, Status and Assessment Report, Proc. 5th Int. Information Exchange Meeting, Mol, Belgium, 1998, NEA/OECD, Paris (1999), pp. 25–27.
a) K. L. Nash, Solvent Extr. Ion Exch., 11, 729 (1993)
K. A. Gschneidner, L. Eyring, G. R. Chopin, and G. H. Lander (eds.), Handbook on the Physics and Chemistry of Rare Earths. Lanthanides/Actinides: Chemistry, Elsevier Science, Amsterdam, (1994), p. 197.
G. Nocton, F. Burdet, J. Pécaut, and M. Mazzanti, Angew. Chem., Int. Ed., 46, 7574–7578 (2007).
a) J. H. Matonic, B. L. Scott, and M. P. Neu, Inorg. Chem., 40, 2638/2639 (2001)
P. Lindqvist-Reis, C. Apostolidis, J. Rebizant, A. Morgenstern, R. Klenze, O. Walter, T. Fanghänel, and R. G. Haire, Angew. Chem. Int. Ed., 46, 919–922 (2007)
S. Skanthakumar, M. R. Antonio, R. E. Wilson, and L. Soderholm, Inorg. Chem., 46, 3485–3491 (2007).
S. A. Cotton, in: Lanthanide and Actinide Chemistry–Inorganic Chemistry, John Wiley & Sons (2006), p. 193.
K. Lyczko et al., Inorg. Chem. Commun., 24, 234–236 (2012).
J. C. Berthet, M. Lance, M. Nierlich, and M. Ephritikhine, J. Inorg. Chem., 2005–2007 (1999).
E. J. Baerends, D. E. Ellis, and P. Ros, Chem. Phys., 2, 41 (1973).
J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh, and C. Fiolhais, Phys. Rev. B, 46, 6671 (1992).
E. Van Lenthe, A. Ehlers, and E. J. Baerends, J. Chem. Phys., 110, 8943 (1999).
D. Hannachi, N. Ouddai, and H. Chermette, Dalton Trans., 39, 3673–3680 (2010).
G. A. Shamov, Inorg. Chem., 51, 6507–6516 (2012)
K. I. M. Ingram, M. J. Tassell, A. J. Gaunt, and N. Kaltsoyannis, Inorg. Chem., 47, 7824–7833 (2008).
A. J. Gaunt, S. D. Reilly, A. E. Enriquez, B. L. Scott, J. A. Ibers, P. Sekar, K. I. M. Ingram, N. Kaltsoyannis, and M. P. Neu, Inorg. Chem., 47, No. 1, 29–41 (2008).
C. R. Graves, E. J. Schelter, T. Cantat, B. L. Scott, and J. L. Kiplinger, Organometallics, 27, 5371–5378 (2008).
J. C. Berthet, M. Nierlich, and M. Ephritikhine, Angew. Chem., Int. Ed., 42, 1952–1954 (2003).
J. C. Berthet, M. Lance, M. Nierlich, and M. Ephritikhine, J. Chem. Soc., Chem. Commun., 1373 (1998).
A. L. Allred and E. G. Rochow, J. Org. Chem., 5, 264 (1949).
S. Lakehal, N. Ouddai, D. Hannachi, and M. Bououdina, Int. J. Quantum Chem., 24339 (2012).
R. G. Pearson, Coord. Chem. Rev., 100, 403 (1990).
P. Geerlings, F. De Proft, and W. Langenaeker, Chem. Rev., 103, 1793 (2003).
P. G. Parr and R. G. Pearson, J. Am. Chem. Soc., 105, 7512 (1983).
R. G. Parr, L. Szentpaly, and S. Liu, J. Am. Chem. Soc., 121, 1922 (1999).
T. Koopmans, Physica, 1, 104 (1933).
S. Liu, J. Chem. Sci., 117, 477 (2005).
K. Lyczko et al., Inorg. Chem. Commun., 24, 234–236 (2012).
A. Khalafi-Nezhad and R. F. Alamdari, Tetrahedron, 57, 6805–6807 (2001).
A. M. Mendoza-Wilson, G. D. Ávila-Quezada, R. R. Balandrán-Quintana, D. Glossman-Mitnik, and S. Ruiz-Cruz, J. Mol. Struct.: THEOCHEM, 897, 6–11 (2009).
D. Marabello, R. Bianchi, G. Gervasio, and F. Cargnoni, Acta Crystallogr., 60, 494 (2004).
R. F. W. Bader and H. J. Essen, J. Chem. Phys., 80, 1943–1960 (1984).
A. Espinosa, L. Alkorta, J. Elguero, and E. Molins, J. Chem. Phys., 117, 5529 (2002).
A. N. Egorova and V. G. Tsirelson, Russ. J. Inorg. Chem., 51, 941 (2006).
B. Carles, M. Costas, J. M. Poblet, and M. Rohmer, et al., Inorg. Chem., 35, 298 (1996).
M. Lein, A. Szabo, A. Kovacs, and G. Frenking, Faraday Discuss., 124, 365–378 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2015 M. Lemmouchi, D. Hannachi, N. Ouddai.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 56, No. 8, pp. 1557-1565, December, 2015.
Rights and permissions
About this article
Cite this article
Lemmouchi, M., Hannachi, D. & Ouddai, N. Comparative study of the lanthanide (Ln) and actinide (An) triflate complexes M(OTf) n . J Struct Chem 56, 1495–1504 (2015). https://doi.org/10.1134/S0022476615080065
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476615080065