Abstract
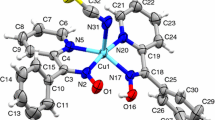
Ligands with Schiff bases are obtained in the condensation of propylenediamine (pda) or 2,2-dimethylpropylenediamine (dmpda) with acetylacetone (Hacac) in the 1:2 molar ratio. The ligands are characterized by the elemental analysis methods, T melt = 90–92 °C for pda(Hacac)2 (pda(acac)2 is N,N′-propylene-bis(acetylacetoniminato) (2-)), T melt = 84–86 °C for dmpda(Hacac)2 (dmpda(acac)2 is N,N′-2,2-dimethylpropylene-bis(acetylacetoniminato) (2-)). The tautomerism of the ligands is established by the single crystal X-ray diffraction (XRD) analysis, IR spectroscopy, and 1H, 13C NMR spectrometry. The synthesized complexes [Cu(pda(acac)2)] (1), T melt = 121–122 °C and [Cu(dmpda(acac)2)] (2), T melt = 156–158 °C are studied by the XRD method. In both complexes, copper atoms have a planar square geometry, and the chelate bond lengths and angles are: Cu-O ≈ Cu-N 1.903(2)–1.942(3) Å, ∠O-Cu-N = 94.44(12)–94.99(12)° for 1 and Cu-O ≈ Cu-N 1.909(1)–1.943(2) Å, ∠O-Cu-N = 94.63(6)° for 2. By the thermogravimetric method it is found that both complexes can be passed practically quantitatively into the gas phase.
Similar content being viewed by others
References
W. H. Leung and C. M. Che, Inorg. Chem., 28, 4116–4120 (1989).
N. S. Enikolopyan, K. A. Bogdanova, and K. A. Askarov, Russ. Chem. Rev., 52, 13–18 (1983).
A. M. El-Hendawy, A. H. Alkubaisi, A. El-Ghany, K. El-Kourashy, and M. N. Sharab, Polyhedron, 12, 2343–2347 (1993).
P. J. McCarthy, R. J. Hovey, K. Ueno, and A. E. Martell, J. Am. Chem. Soc., 77, 5820–5825 (1955).
N. Aksuner, E. Henden, I. Yilmaz, and A. Cukurovali, Dyes Pigm., 83, 211–216 (2009).
Y-P. Cai, C-Y. Su, A-W. Xu, B-S. Kang, Y-X. Tong, H-Q. Liu, and S. Jie, Polyhedron, 20, 657–661 (2001).
G. V. Girichev, N. I. Giricheva, N. P. Kuz’mina, Yu. S. Medvedev, and A. Yu. Rogachev, J. Struct. Chem., 49, No. 5, 837–849 (2008).
N. V. Tverdova, E. D Pelevina., N. I. Giricheva, G. V. Girichev, N. P. Kuzmina, and O. V. Kotova, J. Mol. Struct., 1012, 151–161 (2012).
G. V. Girichev, N. I. Giricheva, N. P. Kuz’mina, and O. V. Kotova, J. Struct. Chem., 50, No. 1, 52–59 (2009).
R. C. Charles, J. Phys. Chem., 65, No. 3, 3383–3390 (1961).
Yu. V. Chumachenko, Chem. Sc. Candidate’s Diss., 02.00.01, Novosibirsk (1979).
G. I. Zharkova, S. I. Dorovskikh, S. V. Sysoev, I. P. Asanov, A. V. Panin, N. B. Morozova, and I. K. Igumenov, Surf. Coat. Techn., 230, 290–296 (2013).
R. G. Charles, Thermochim. Acta, 38, 315–327 (1980).
P. A. Premkumar, R. Pankajavalli, O. M. Sreedharan, V. S. Raghunathan, K. S. Nagaraja, and C. Mallika, Mater. Lett., 58, 2256–2260 (2004).
S. Arockiasamy, P. A. Premkumar, R. Pankajavalli, O. M. Sreedharan, V. S. Raghunathan, K. S. Nagaraja, and C. Mallika, J. Mater. Sci., 41, 3383–3390 (2006).
APEX2 (Version 1.08), SAINT (Version 7.03), and SADABS (Version 2.11). Bruker Advanced X-ray Solutions, Bruker AXS Inc., Madison, Wisconsin, USA (2004).
G. M. Sheldrick, Acta Crystallogr., A64, No. 1, 112–122 (2008).
Yue-Peng Cai, Cheng-Yong Su, An-wu Xu, Bei-Sheng Kang, Ye-Xiang Tong, Han-Qin Liu, and Sun Jie, Polyhedron, 20, 657–662 (2001).
A. E. Martell, R. N. Belford, and M. Calvin, J. Inorg. Nucl. Chem., 5, 170–176 (1958).
S. I. Dorovskikh, A. V. Alexeyev, N. V. Kuratieva, T. V. Basova, V. G. Kiselev, L. A. Sheludyakova, Yu. V. Shubin, N. B. Morozova, and I. K. Igumenov, J. Organomet. Chem.; doi.org/10.1016/j.jorganchem.2013.05.001.
G. C. Percy and D. A. Thornton, J. Inorg. Nucl. Chem., 34, 3357–3362 (1972).
D. N. Kendall, Applied Infrared Spectroscopy, Reinhold Publishing, New York (1966).
S. M. Abn-EI-Wafa, R. A. Issa, and C. A. McAuliffe, Inorg. Chim. Acta, 99, 103–113 (1985).
W. S. Rees and C. R. Caballero, Adv. Mater. Optic. Electron., 1, 59–63 (1992).
I. A. Baidina, P. A. Stabnikov, A. D. Vasilèv, S. A. Gromilov, and I. K. Igumenov, J. Struct. Chem., 45, No. 4, 671–677 (2004).
P. A. Stabnikov, G. I. Zharkova, I. A. Baidina, S. V. Tkachev, V. V. Krisyuk, and I. K. Igumenov, Polyhedron, 26, 4445–4449 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2014 S. I. Dorovskikh, N. V. Kuratieva, S. V. Tkachev, S. V. Trubin, P. A. Stabnikov, N. B. Morozova.
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 55, No. 6, pp. 1124–1131, November–December, 2014.
Rights and permissions
About this article
Cite this article
Dorovskikh, S.I., Kuratieva, N.V., Tkachev, S.V. et al. Copper(II) complexes with Schiff bases: Structures and thermal behavior. J Struct Chem 55, 1067–1074 (2014). https://doi.org/10.1134/S0022476614060092
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476614060092



