Abstract
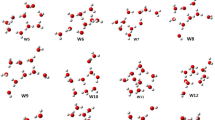
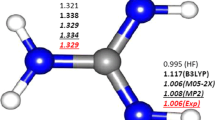
The B3LYP method within DFT and the ab initio MP2 method with an extended 6-311++G(3df,3pd) basis set are employed to calculate the adiabatic bound state of an excess electron in (H2O) −6 water and (NH3) −13 . ammonium clusters. Adiabatic electron affinity of (H2O)6 and (NH3)13 clusters is 0.03–0.18 eV and 0.18 eV respectively. The calculated vertical binding energies of the excess electron in anionic clusters ((H2O) −6 0.37÷0.66 eV and (NH3) −13 0.26 eV) agree well with the experimental values of 0.50 eV and 0.22 eV obtained from photoelectron spectra. A cavity model of solvated electrons in water and ammonium is considered.
Similar content being viewed by others
References
E. Zurek, P. P. Edwards, and R. Hoffman, Angew. Chem. Int. Ed., 48, 8198–8232 (2009).
A. M. Brodskii and A. V. Tsarevskii, Usp. Khim., 56, No. 10, 1693–1716 (1987).
I. A. Shkrob, J. Phys. Chem. A, 110, 3967–3976 (2006).
I. A. Shkrob, J. Phys. Chem. A, 111, 5223–5231 (2007).
R. A. Ogg, Phys. Rev., 69, 668/669 (1946).
I.-R. Lee, W. Lee, and A. H. Zewail, Chem. Phys. Chem., 9, 83–88 (2008).
H. W. Sarkas, S. T. Arnold, J. G. Eaton, et al., J. Chem. Phys., 116, 5731–5737 (2002).
J. V. Coe, G. H. Lee, J. G. Eaton, et al., J. Chem. Phys., 92, 3980–3982 (1990).
P. Krebs, J. Phys. Chem., 88, 3702–3709 (1984).
T. Sommerfeld, J. Phys. Chem. A, 112, 11817–11823 (2008).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785–797 (1988).
M. Head-Gordon, J. A. Pople, and M. J. Frisch, Chem. Phys. Lett., 153, 503–506 (1988).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al. Gaussian 03, Revision B.02, Gaussian, Inc., Pittsburgh PA (2003).
U. Schindewolf and P. Wunschel, Canad. J. Chem., 55, 2159–2164 (1977).
A. W. Castleman and K. H. Bowen, J. Phys. Chem., 100, 12911–12944 (1996).
J. M. Herbert and M. Head-Gordon, Proc. Natl. Acad. Sci. USA, 103, No. 39, 14282–14287 (2006).
E. J. Hart and M. Anbar, The Hydrated Electron, Wiley-Interscience, New York (1970).
J. C. Thompson, Electrons in Liquid Ammonia, Clarendon Press, Oxford (1976).
T. Takayanagi, T. Yoshikawa, H. Motegi, and M. Shiga, Chem. Phys. Lett., 482, Nos. 4–6, 195–200 (2009).
Yu. V. Novakovskaya, Zh. Fiz. Khim., 84, No. 2, 291–306 (2010).
G. Lepoutre and J. Jotner, J. Phys. Chem., 76, 683–687 (1972).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2014 I. I. Zakharov.
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 55, No. 1, pp. 7–13, January–February, 2014.
Rights and permissions
About this article
Cite this article
Zakharov, I.I. Structure of water (H2O) −6 and ammonium (NH3) −13 clusters with an excess electron: Quantum chemical studies. J Struct Chem 55, 1–7 (2014). https://doi.org/10.1134/S0022476614010016
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476614010016