Abstract
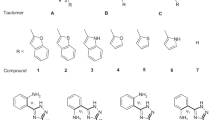
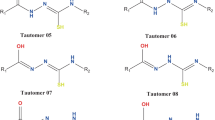
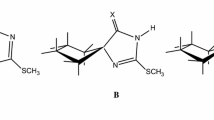
Thioimidazoline derivatives can be used to treat hyperthyroidism due to their ability to make complexes with iodine. In this research designed to find new structures with the same ability, 1-methyl-2-thioxoimidazolidin-4-one (MTIO) and the structures of MTIO tautomers (5 tautomers), their isomers (total 9 isomers) and their complexes with iodine are optimized using the B3LYP method with two different basis sets to obtain their molecular parameters, relative energies, and vibrational frequencies. The relative energies show that in all tautomers and complexes, ketone and thione forms are more stable than enol and thienol forms, and also Z isomers are more stable than E isomers. Moreover, the NBO calculation is carried out for tautomers and complexes to obtain atomic charges and acceptor-donor interactions. These results confirm the ability of MTIO tautomers to form complexes and show that the planar complexes have more effective interaction than the perpendicular complexes. The essence and important complexation properties are also calculated and confirmed using the AIM analysis.
Similar content being viewed by others
References
H. Kohn, B. A. Kohn, M. L. Steenberg, and J. P. Buckley, J. Med. Chem., 20, 58–64 (1977).
C. Laurence, M. J. Elghomari, and M. Lucon, J. Chem. Soc. Perkin Trans. 2, 1159–1162 (1998).
C. Laurence, M. J. Elghomari, and M. Berthelot, J. Chem. Soc. Perkin Trans. 2, 1163–1167 (1998).
C. Laurence, M. J. Elghomari, J. Y. Lequestel, M. Berthelo, and R. Mokhlisse, J. Chem. Soc. Perkin Trans. 2, 1545–1551 (1998).
H. Roohi, A. Ebrahimi, and S. M. Habibi, Theochem., 710, 77–82 (2004).
D. K. Papayannis and A. M. Kosmas, Theochem., 851, 175–179 (2008).
A. Taurog, J. Biochem. Biophys., 24, 330–337 (1996).
E. S. Raper, J. R. Creighton, R. E. Oughtred, and I. W. Nowell, Acta Cryst. B, 39, 355–361 (1983).
C. Laurence, M. J. Elghomari, J. Y. Lequestel, M. Berthelot, and R. Mokhisse, J. Chem. Soc. Perkin Trans. 2, 1553–1557 (1998).
B. Jemec, Acta Pathol. Microbiol. Scand. A, 78, 151–155 (1970).
G. Roy and G. Mugesh, J. Chem. Sci., 118, 619–624 (2006).
G. Roy and G. Mugesh, J. Inorg. Chem. Acta., 360, 303–308 (2000).
G. Roy and G. Mugesh, J. Inorg. Phys. Chem., 1–6 (2006).
J. Kohrle, Endor. Rev., 23, 944–948 (2005).
H. B. Dunford, Biochem., 445, 199–204 (2006).
J. Kohrle, Exp. Clin. Endocrinol., 102, 63–67 (1994).
M. J. Berry, L. Banu, and P. R. Larse, Nature, 349, 348–354 (1991).
A. C. Bianco, D. Salvatore, B. Gereben, M. J. Berry, and P. R. Larsen, Endocrine Rev., 23, 38–45 (2002).
J. Kohrle, Biochimie, 81, 527–534 (1999).
M. J. Nowac, J. Phys. Chem., 94, 7406–7411 (1990).
A. Fu, Theochem., 767, 510–513 (2006).
N. V. Belova, H. Oberhammer, G. V. Girichev, and S. A. Shlykov, J. Phys. Chem. A, 112, 3209–3216 (2008).
H. Tavakol, Theochem., 954, 16–21 (2010).
R. Dobosz, E. Kolehmainen, A. Valkonen, B. Osmiaowski, and R. Gawinecki, Tetrahedron, 63, 9172–9178 (2007).
H. Tavakol, Theochem., 916, 172–179 (2009).
A. Misra and S. Dalai, Theochem., 807, 33–37 (2007).
H. Tavakol and H. Sabzyan, J. Phys. Org. Chem., 1771–1776 (2010).
H. Tavakol and S. Arshadi, J. Mol. Model., 15, 807–816 (2009).
A. D. Dubonosov, V. I. Minkin, V. A. Bren, E. N. Shepelenko, A. V. Tsukanov, A. G. Starikov, and G. S. Borodkin, Tetrahedron, 64, 3160 (2008).
H. Tavakol, Mol. Simul., 36, 391–402 (2010).
B. I. Buzykin, E. V. Mronova, V. N. Nabiullin, N. M. Azancheev, L. V. Awakumova, I. K. Rizvanov, T. Gubaiduffin, I. A. Litvinov, and V. V. Syakaev, Russ. J. Gen. Chem., 78, 461–468 (2008).
H. Tavakol, Int. J. Quant. Chem., QUA 22847 (2010).
J. A. Bonacin, D. Melo, and H. E. Toma, Vib. Spectroscop., 107 (2007).
H. Tavakol, Theochem., 956, 97–102 (2010).
A. Bhan, Y. V. Joshi, W. N. Delgass, and K. T. Thomson, J. Phys. Chem. B, 107, 10476–10484 (2003).
X. Rozanska, R. A. Santen, T. Demuth, F. Hutschka, and J. Hafner, J. Phys. Chem. B., 107, 1309–1312 (2003).
A. D. Becke, J. Chem. Phys., 98, 5648–5653 (1993).
T. C. Lee, W. T. Yang, and R. G. Parr, Phys. Rev. B., 37, 785–791 (1988).
B. G. Johnson, P. M. W. Gill, and J. A. Pople, J. Chem. Phys., 98, 5612–5616 (1993).
C. W. Bauschlicher and H. Partridge, J. Chem. Phys., 103, 1788–1794 (1995).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785–793 (1988).
A. E. Reed, L. A. Curtiss, and F. Weinhold, Chem. Rev., 88, 899–903 (1988).
M. J. Frisch et al., Gaussian 98 Rev. A.1, Gaussian Inc., Pittsburgh PA (1998).
A. P. Scott and I. Radom, J. Phys. Chem. B, 100, 16502–16508 (1996).
F. Bieglerkonig and J. Schonbohm, J. Comp. Chem., 20–24 (2002).
R. F. W. Bader, Atoms in Molecules. A Quantum Theory, Oxford University Press, New York (1990).
A. Alparone, A. Millefiori and S. Millefio, Chem. Phys., 312, 261–267 (2005).
A. F. Jalbout, B. Trzaskowski, Y. Xia, Y. Li, X. Hu, and H. Li, Chem. Phys., 332, 152–157 (2007).
A. Elnahas, L. A. W. Liang, H. Li, X. Hu, and S. Han, Chem. Phys., 328, 93–97 (2006).
F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry, 5th ed., Springer, US, New York (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2012 by H. Tavakol, T. Hadadi, H. Roohi
__________
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 53, No. 4, pp. 662–671, July–August, 2012.
Rights and permissions
About this article
Cite this article
Tavakol, H., Hadadi, T. & Roohi, H. DFT, AIM, and NBO analyses of 1-methyl-2-thioxoimidazolidin-4-one tautomers and their complexes with iodine. J Struct Chem 53, 649–658 (2012). https://doi.org/10.1134/S0022476612040063
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476612040063