Abstract
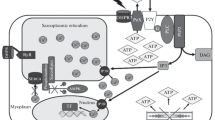
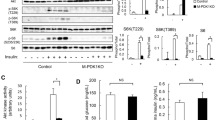
Skeletal muscle atrophy during their unloading is due to a decrease in protein synthesis and an increase in proteolysis. The accumulation of ATP in muscle during unloading, detected at its early stages, may be one of the stimuli triggering this process. It has been shown that pannexin channels allow ATP efflux from the cytoplasm to the extracellular space during muscle unloading. Extracellular ATP can be sensed by P2Y2 receptors. To test the hypothesis on the involvement of P2Y2 receptors in the regulation of signaling processes in skeletal muscles at early stages of unloading, they were inhibited by their selective inhibitor AR-C 18925XX. The inhibition of P2Y2 receptors during 3-day unloading attenuated m. soleus atrophy, prevented ATP accumulation therein, downregulated the expression of MAFbx E3-ligase mRNA, ubiquitin and IL6 receptors, upregulated the level of AMPK phosphorylation and intensity of protein synthesis.










Similar content being viewed by others
REFERENCES
Baldwin KM, Haddad F (2002) Skeletal muscle plasticity: cellular and molecular responses to altered physical activity paradigms. Am J Phys Med Rehabil 81 (11 Suppl): S40–S51. https://doi.org/10.1097/01.PHM.0000029723.36419.0D
Fitts RH, Riley DR, Widrick JJ (2000) Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol 89 (2): 823–839. https://doi.org/10.1152/jappl.2000.89.2.823
Fluck M, Hoppeler H (2003) Molecular basis of skeletal muscle plasticity-from gene to form and function. Rev Physiol Biochem Pharmacol 146: 159–216. https://doi.org/10.1007/s10254-002-0004-7
Hodson N, West DWD, Philp A, Burd NA, Moore DR (2019) Molecular regulation of human skeletal muscle protein synthesis in response to exercise and nutrients: a compass for overcoming age-related anabolic resistance. Am J Physiol Cell Physiol 317 (6): C1061–C1078. https://doi.org/10.1152/ajpcell.00209.2019
Belova SP, Tyganov SA, Mochalova EP, Shenkman BS (2021) Restricted Activity and Protein Synthesis in Postural and Locomotor Muscles. Russ J Physiol 107: 842–853. https://doi.org/10.1134/S0022093021030194
Zaripova KA, Kalashnikova EP, Belova SP, Kostrominova TY, Shenkman BS, Nemirovskaya TL (2021) Role of Pannexin 1 ATP-Permeable Channels in the Regulation of Signaling Pathways during Skeletal Muscle Unloading. Int J Mol Sci 22 (19): 10444. https://doi.org/10.3390/ijms221910444
Shenkman BS (2020) How Postural Muscle Senses Disuse? Early Signs and Signals. Int J Mol Sci 21 (14): 5037. https://doi.org/10.3390/ijms21145037
Ohira Y, Yasui W, Kariya F, Wakatsuki T, Nakamura K, Asakura T, Edgerton VR (1994) Metabolic adaptation of skeletal muscles to gravitational unloading. Acta Astronaut 33: 113–117. https://doi.org/10.1016/0094-5765(94)90115-5
Gupta RC, Misulis KE, Dettbarn WD (1989) Activity dependent characteristics of fast and slow muscle: biochemical and histochemical considerations. Neurochem Res 14 (7): 647–655. https://doi.org/10.1007/BF00964874
Casas M, Buvinic S, Jaimovich E (2014) ATP signaling in skeletal muscle: from fiber plasticity to regulation of metabolism. Exerc Sport Sci Rev 42 (3): 110–116. https://doi.org/10.1249/JES.0000000000000017
Dahl G (2015) ATP release through pannexon channels. Philos Trans R Soc Lond B Biol Sci 370 (1672): 10.1098/rstb.2014.0191.
May C, Weigl L, Karel A, Hohenegger M (2006) Extracellular ATP activates ERK1/ERK2 via a metabotropic P2Y1 receptor in a Ca2+ independent manner in differentiated human skeletal muscle cells. Biochem Pharmacol 71 (10): 1497–1509. https://doi.org/10.1016/j.bcp.2006.02.003
Arias-Calderon M, Almarza G, Diaz-Vegas A, Contreras-Ferrat A, Valladares D, Casas M, Toledo H, Jaimovich E, Buvinic S (2016) Characterization of a multiprotein complex involved in excitation-transcription coupling of skeletal muscle. Skelet Muscle 6: 15. https://doi.org/10.1186/s13395-016-0087-5
Riquelme MA, Cea LA, Vega JL, Boric MP, Monyer H, Bennett MV, Frank M, Willecke K, Saez JC (2013) The ATP required for potentiation of skeletal muscle contraction is released via pannexin hemichannels. Neuropharmacology 75: 594–603. https://doi.org/10.1016/j.neuropharm.2013.03.022
Liu B, Cao W, Li J, Liu J (2018) Lysosomal exocytosis of ATP is coupled to P2Y2 receptor in marginal cells in the stria vascular in neonatal rats. Cell Calcium 76: 62–71. https://doi.org/10.1016/j.ceca.2018.09.006
Morey-Holton E, Globus RK, Kaplansky A, Durnova G (2005) The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv Space Biol Med 10: 7–40. https://doi.org/10.1016/s1569-2574(05)10002-1
Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, Hornberger TA (2011) Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J 25 (3): 1028–1039. https://doi.org/10.1096/fj.10-168799
Ravi V, Jain A, Mishra S, Sundaresan NR (2020) Measuring Protein Synthesis in Cultured Cells and Mouse Tissues Using the Non-radioactive SUnSET Assay. Curr Protoc Mol Biol 133 (1): e127. https://doi.org/10.1002/cpmb.127
Chen M, Chen H, Gu Y, Sun P, Sun J, Yu H, Zheng H, Chen D (2021) P2Y2 promotes fibroblasts activation and skeletal muscle fibrosis through AKT, ERK, and PKC. BMC Musculoskelet Disord 22 (1): 680. https://doi.org/10.1186/s12891-021-04569-y
Ito N, Ruegg UT, Takeda S (2018) ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy. Int J Mol Sci 19 (9): 2804. https://doi.org/10.3390/ijms19092804
Vilchinskaya NA, Mochalova EP, Nemirovskaya TL, Mirzoev TM, Turtikova OV, Shenkman BS (2017) Rapid decline in MyHC I(beta) mRNA expression in rat soleus during hindlimb unloading is associated with AMPK dephosphorylation. J Physiol (London) 595 (23): 7123–7134. https://doi.org/10.1113/JP275184
Tyganov SA, Mochalova EP, Belova SP, Sharlo KA, Rozhkov SV, Vilchinskaya NA, Paramonova II, Mirzoev TM, Shenkman BS (2019) Effects of Plantar Mechanical Stimulation on Anabolic and Catabolic Signaling in Rat Postural Muscle Under Short-Term Simulated Gravitational Unloading. Front Physiol 10: 1252. https://doi.org/10.3389/fphys.2019.01252
Stouth DW, Manta A, Ljubicic V (2018) Protein arginine methyltransferase expression, localization, and activity during disuse-induced skeletal muscle plasticity. Am J Physiol Cell Physiol 314 (2): C177–C190. https://doi.org/10.1152/ajpcell.00174.2017
Stouth DW, vanLieshout TL, Ng SY, Webb EK, Manta A, Moll Z, Ljubicic V (2020) CARM1 Regulates AMPK Signaling in Skeletal Muscle. iScience 23 (11): 101755. https://doi.org/10.1016/j.isci.2020.101755
Mathew TS, Ferris RK, Downs RM, Kinsey ST, Baumgarner BL (2014) Caffeine promotes autophagy in skeletal muscle cells by increasing the calcium-dependent activation of AMP-activated protein kinase. Biochem Biophys Res Commun 453 (3): 411–418. https://doi.org/10.1016/j.bbrc.2014.09.094
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294 (5547): 1704–1708. https://doi.org/10.1126/science.1065874
Glass DJ (2003) Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5 (2): 87–90. https://doi.org/10.1038/ncb0203-87
Yakabe M, Ogawa S, Ota H, Iijima K, Eto M, Ouchi Y, Akishita M (2018) Inhibition of interleukin-6 decreases atrogene expression and ameliorates tail suspension-induced skeletal muscle atrophy. PLoS One 13 (1): e0191318. https://doi.org/10.1371/journal.pone.0191318
Sun H, Sun J, Li M, Qian L, Zhang L, Huang Z, Shen Y, Law BY, Liu L, Gu X (2021) Transcriptome Analysis of Immune Receptor Activation and Energy Metabolism Reduction as the Underlying Mechanisms in Interleukin-6-Induced Skeletal Muscle Atrophy. Front Immunol 12: 730070. https://doi.org/10.3389/fimmu.2021.730070
Hodge DR, Cho E, Copeland TD, Guszczynski T, Yang E, Seth AK, Farrar WL (2007) IL-6 enhances the nuclear translocation of DNA cytosine-5-methyltransferase 1 (DNMT1) via phosphorylation of the nuclear localization sequence by the AKT kinase. Cancer Genomics Proteomics 4 (6): 387–398.
Hodson N, Philp A (2019) The Importance of mTOR Trafficking for Human Skeletal Muscle Translational Control. Exerc Sport Sci Rev 47 (1): 46–53. https://doi.org/10.1249/JES.0000000000000173
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75 (1): 50–83. https://doi.org/10.1128/MMBR.00031-10
Yu M, Blomstrand E, Chibalin AV, Krook A, Zierath JR (2001) Marathon running increases ERK1/2 and p38 MAP kinase signalling to downstream targets in human skeletal muscle. J Physiol 536 (Pt 1): 273–282. https://doi.org/10.1111/j.1469-7793.2001.00273.x
Mirzoev TM, Shenkman BS (2018) Regulation of Protein Synthesis in Inactivated Skeletal Muscle: Signal Inputs, Protein Kinase Cascades, and Ribosome Biogenesis. Biochemistry (Mosc) 83 (11): 1299–1317. https://doi.org/10.1134/S0006297918110020
Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68 (2): 320–344. https://doi.org/10.1128/MMBR.68.2.320-344.2004
Kimball SR, Jefferson LS (2006) Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr 136 (1 Suppl): 227S–231S. https://doi.org/10.1093/jn/136.1.227S
Carriere A, Ray H, Blenis J, Roux PP (2008) The RSK factors of activating the Ras/MAPK signaling cascade. Front Biosci 13: 4258–4275. https://doi.org/10.2741/3003
Carriere A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, Roux PP (2008) Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol 18 (17): 1269–1277. https://doi.org/10.1016/j.cub.2008.07.078
Funding
This study was supported be the Russian Foundation for Basic Research (RFBR), grant no. 20-015-00138.
Author information
Authors and Affiliations
Contributions
Conceptualization, experimental design, writing and editing of the manuscript (L.N.T.); experimental design, data collection and processing, writing and editing of the manuscript (S.P.B.); data collection and processing, editing of the manuscript (K.A.Z.); general supervision (B.S.Sh.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have neither evident nor potential conflict of interest related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2022, Vol. 58, No. 6, pp. 487–497https://doi.org/10.31857/S0044452922060134.
Rights and permissions
About this article
Cite this article
Zaripova, K.А., Belova, S.P., Shenkman, B.S. et al. The Role of P2Y Receptors in the Regulation of Atrophic Processes in Rat Skeletal Muscles under Unloading. J Evol Biochem Phys 58, 1708–1719 (2022). https://doi.org/10.1134/S0022093022060047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022060047