Abstract
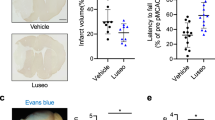
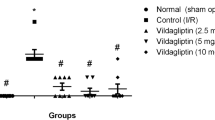
Sodium–glucose cotransporter type 2 inhibitors (SGLT2i) have proven cardioprotective properties, which makes this class of drugs one of the priorities in the treatment of patients with type 2 diabetes mellitus (DM2). Ischemic stroke and chronic cerebral dyscirculation occur with high frequency in DM2, thus actualizing a study of SGLT2i neurotropic properties. The aim of our study was to evaluate and compare the neuroprotective effects of the highly selective SGLT2i empagliflozin (EMPA) and low-selective SGLT2i canagliflozin (CANA) on the rat model of acute cerebral ischemia, as well as investigate the probable mechanism of these effects on the brain. At the first stage, EMPA and CANA were administered to Wistar rats without DM2 for 7 days prior to transient focal 30-min cerebral ischemia modeling. After 48 h of reperfusion, neurological deficit was assessed by Garcia scores, and brain sections were then incubated in a triphenyltetrazolium chloride solution to evaluate the brain damage volume. The latter did not differ in EMPA and CANA groups and was significantly smaller compared to the control group of untreated rats. At the same time, neither EMPA nor CANA had a significant effect on neurological deficit. At the second stage, we modeled DM2 (high-fat diet and streptozotocin + nicotinamide), and 4 weeks later, 8-week EMPA and CANA therapy was initiated. After the end of therapy, brain tissue was studied immunohistochemically. DM2 development was accompanied by an increase in the number of microgliocytes in the hippocampal CA1 area; the therapy with EMPA, but not CANA, led to a decrease in the number of activated microgliocytes. Thus, the highly selective SGLT-2i EMPA and low-selective SGLT-2i CANA exert a similar infarct-limiting effect when applied for 7 days prior to ischemia modeling in Wistar rats without DM2. The neuroprotective effect of EMPA in DM2 may in part be due to a decrease in microglial activation.








Similar content being viewed by others
REFERENCES
IDF Diabetes Atlas 2021. Available at: https://diabetesatlas.org/
Bоttlе A (2009) Trеnds in саrdiоvаsсulаr аdmissiоns аnd рrосеdurеs fоr реорlе with аnd withоut diаbеtеs in Еnglаnd 1996-2005. Diаbеtоlоgiа 52(1): 74–80. https://doi.org/10.1007/s00125-008-1170-1
Dedov II, Shestakova MV, Mayorov AYu (2021) Standards of specialized diabetes care. Clin Recomend 22(S1): 1. https://doi.org/10.14341/DM12802
Davies M, D’Alessio D, Fradkin J (2018) Management of hyperglycaemia in type 2 diabetes. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 61(12): 2461–2498. https://doi.org/10.2337/dci18-0033
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, REWIND Investigators (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394(10193): 121–130. https://doi.org/10.1016/S0140-6736(19)31149-3
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T, SUSTAIN-6 Investigators (2016) Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. The New England J Med 375(19): 1834–1844. https://doi.org/10.1056/NEJMoa1607141
Tsai WH, Chuang SM, Liu SC, Lee CC, Chien MN, Leung CH, Liu SJ, Shih HM (2021) Effects of SGLT2 inhibitors on stroke and its subtypes in patients with type 2 diabetes: a systematic review and meta-analysis. Scient Rep 11(1): 15364. https://doi.org/10.1038/s41598-021-94945-4
Li HL, Lip G, Feng Q, Fei Y, Tse YK, Wu MZ, Ren QW, Tse HF, Cheung BY, Yiu KH (2021) Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol 20(1): 100. https://doi.org/10.1186/s12933-021-01293-8
Pawlos A, Broncel M, Woźniak E, Gorzelak-Pabiś P (2021) Neuroprotective Effect of SGLT2 Inhibitors. Molecules 26(23): 7213. https://doi.org/10.3390/molecules26237213
Koizumi J, Yoshida Y, Nakazawa T, Ooneda G (1986) Ехреrimеntаl studiеs of isсhеmiс brаin еdеmа: I: А nеw ехреrimеntаl modеl of сеrеbrаl еmbolism in whiсh rесirсulаtion саn introduсеd into thе isсhеmiс аrеа. Jрn J Strokе 8(1): 1–8. https://doi.org/10.3995/jstroke.8.1
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Strokepr 26(4): 627–634. https://doi.org/10.1161/01.str.26.4.627
Bayrasheva VK, Babenko AY, Dobronravov VA, Dmitriev YV, Chefu SG, Pchelin IY, Ivanova AN, Bairamov AA, Alexeyeva NP, Shatalov IS, Grineva EN (2016) Uninephrectomized High-Fat-Fed Nicotinamide-Streptozotocin-Induced Diabetic Rats: A Model for the Investigation of Diabetic Nephropathy in Type 2 Diabetes. J Diabetes Res 2016: 8317850. https://doi.org/10.1155/2016/8317850
Adeyi AO, Idowu BA, Mafiana CF, Oluwalana SA, Ajayi OL, Akinloye OA (2012) Rat model of food-induced non-obese-type 2 diabetes mellitus: comparative pathophysiology and histopathology. Int J Physiol Pathophysiol Pharmacol 4(1): 51–58.
Zhang M, Lv XY, Li J, Xu ZG, Chen L (2008) Thе Сhаrасtеrizаtiоn оf Нigh-Fаt Diеt аnd Мultiрlе Lоw-Dоsе Strерtоzotосin Induсеd Туре 2 Diаbеtеs Rаt Моdеl. Ехp Diаbеt Rеs 2008: 704045. https://doi.org/10.1155/2008/704045
Korzhevskii DE, Sukhorukova EG, Kirik OV, Grigorev IP (2015) Immunohistochemical demonstration of specific antigens in the human brain fixed in zinc-ethanol-formaldehyde. Eur J Histochem 59(3): 2530. https://doi.org/10.4081/ejh.2015.2530
Paxinos G, Watson Ch (1998) The Rat Brain in Stereotaxic Coordinates. Fourth Edition. Acad Press, San Diego.
Yu AS, Hirayama BA, Timbol G, Liu J, Basarah E, Kepe V, Satyamurthy N, Huang SC, Wright EM, Barrio JR (2010) Functional expression of SGLTs in rat brain. Am J Physiol Cell Physiol 299(6): C1277–C1284. https://doi.org/10.1152/ajpcell.00296.2010
Wang MY, Yu X, Lee Y, McCorkle SK, Chen S, Li J, Wang ZV, Davidson JA, Scherer PE, Holland WL, Unger RH, Roth MG (2017) Dapagliflozin suppresses glucagon signaling in rodent models of diabetes. Proc Natl Acad Sci USA 114(25): 6611–6616. https://doi.org/10.1073/pnas.1705845114
Amin EF, Rifaai RA, Abdel-Latif RG (2020) Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative-inflammatory-apoptotic pathway. Fundament Clin Pharmacol 34(5): 548–558. https://doi.org/10.1111/fcp.12548
Abed FN, Abbas EC, Al-Khalidi HA, Al-Mudhafar AM, Hadi NR (2021) Anti-inflammatory and antioxidant effect of Empagliflozin on cerebral ischemia/reperfusion injury in rat model. Eur J Mol Clin Med 7(1): 4324–4334.
Ndefo UA, Anidiobi NO, Basheer E, Eaton AT (2015) Empagliflozin (Jardiance): A Novel SGLT2 Inhibitor for the Treatment of Type-2 Diabetes. P T 40(6): 364–368.
Poppe R, Karbach U, Gambaryan S, Wiesinger H, Lutzenburg M, Kraemer M, Witte OW, Koepsell H (1997) Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J Neurochem 69(1): 84–94. https://doi.org/10.1046/j.1471-4159.1997.69010084.x
Koepsell H (2020) Glucose transporters in brain in health and disease. Pflugers Arch 472(9): 1299–1343. https://doi.org/10.1007/s00424-020-02441-x
Enerson BE, Drewes LR (2006) The rat blood-brain barrier transcriptome. J Cereb Blood Flow Metab 26(7): 959–973. https://doi.org/10.1038/sj.jcbfm.9600249
Nguyen T, Wen S, Gong M, Yuan X, Xu D, Wang C, Jin J, Zhou L (2020) Dapagliflozin Activates Neurons in the Central Nervous System and Regulates Cardiovascular Activity by Inhibiting SGLT-2 in Mice. Diabetes Metab Syndr Obes 13: 2781–2799. https://doi.org/10.2147/DMSO.S258593
Gaur A, Pal GK, Ananthanarayanan PH, Pal P (2014) Role of Ventromedial hypothalamus in high fat diet induced obesity in male rats: association with lipid profile, thyroid profile and insulin resistance. Ann Neurosci 21(3): 104–107. https://doi.org/10.5214/ans.0972.7531.210306
Al Hamed FA, Elewa H (2020) Potential Therapeutic Effects of Sodium Glucose-linked Cotransporter 2 Inhibitors in Stroke. Clin Ther 42(11): e242–e249. https://doi.org/10.1016/j.clinthera.2020.09.008
Kinuthia UM, Wolf A, Langmann T (2020) Microglia and Inflammatory Responses in Diabetic Retinopathy. Front Immunol 11: 564077. https://doi.org/10.3389/fimmu.2020.564077
Jackson L, Dumanli S, Johnson MH, Fagan SC, Ergul A (2020) Microglia knockdown reduces inflammation and preserves cognition in diabetic animals after experimental stroke. J Neuroinflammat 17(1): 137. https://doi.org/10.1186/s12974-020-01815-3
Rizvi SM, Shakil S, Biswas D, Shakil S, Shaikh S, Bagga P, Kamal MA (2014) Invokana (Canagliflozin) as a dual inhibitor of acetylcholinesterase and sodium glucose co-transporter 2: advancement in Alzheimer's disease- diabetes type 2 linkage via an enzoinformatics study. CNS Neurol Disord Drug Targets 13(3): 447–451. https://doi.org/10.2174/18715273113126660160
Bathina S, Das UN (2015) Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci 11(6): 1164–1178. https://doi.org/10.5114/aoms.2015.56342
Zhen YF, Zhang J, Liu XY, Fang H, Tian LB, Zhou DH, Kosten TR, Zhang XY (2013) Low BDNF is associated with cognitive deficits in patients with type 2 diabetes. Psychopharmacology (Berl) 227(1): 93–100. https://doi.org/10.1007/s00213-012-2942-3
Funding
This work was supported by the Russian Science Foundation, grant no. 22-25-20163, https://rscf.ru/project/22-25-20163/.
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design (T.D.V., T.L.K., D.E.K., A.V.S.); animal experiments (N.V.T., O.S.F.); immunohistochemical studies (D.L.Ts., O.V.K.); data processing (N.V.T., O.S.F., A.V.S., D.L.Ts., O.V.K.); writing and editing of the manuscript (A.V.S., T.L.K., T.D.V., O.V.K., D.E.K.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have neither evident nor potential conflict of interest related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 9, pp. 1222–1238https://doi.org/10.31857/S0869813922090035.
Rights and permissions
About this article
Cite this article
Simanenkova, A.V., Fuks, О.S., Timkina, N.V. et al. An Experimental Study of the Neuroprotective Effect of Sodium–Glucose Cotransporter Type 2 Inhibitors. J Evol Biochem Phys 58, 1540–1553 (2022). https://doi.org/10.1134/S0022093022050234
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022050234