Abstract
Trace amines (TA) are a family of endogenous compounds structurally similar to classical biogenic amines that may be involved in the pathogenesis of a number of neuropsychiatric disorders. One of the most studied and perspective member of the TA associated receptors (TAARs) family is the TAAR1. The aim of the present study was to investigate the sensory gating (SG) in freely moving TAAR1 knockout mice in a chronic experiment. The study of SG was conducted in the paired-click paradigm. The SG indices were calculated as an absolute value by subtracting the second stimulus response amplitude from the first stimulus response amplitude (S1–S2) and as a relative value calculated by dividing the S2 amplitude by the response amplitude on S1 (S2/S1). As a result, a significant decrease in the amplitude of the N40 component was found in TAAR1 knockout mice compared to wild-type mice. In addition, the absolute value of sensory gating calculated by the S1–S2 method was also reduced, but the relative value of sensory gating denoted as S1/S2 ratio remained unchanged. Thus, the data obtained indicate the involvement of TAAR1 in the generation of auditory evoked potentials and the potential involvement of the trace amine system in the dosing and filtering of sensory information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Trace amines (TA) are a family of endogenous compounds structurally similar to classical biogenic amines. Trace amines are found in mammalian tissues at nanomolar concentrations and can be considered as potential neuromodulators [1, 2]. In particular, the effect of TA on dopaminergic [3, 4], serotonergic [3] and glutamatergic transmission [5, 6] has been shown.
The most studied member of the TAARs (trace amines associated receptors) family is the TAAR1, which is found in the central nervous system and peripheral tissues [7]. The TAAR1 receptor is expressed mainly in the most significant concentrations in the regions of dopaminergic neurons highest density, namely in the ventral tegmental area (VTA) and substantia nigra (SN), in the dorsal raphe nucleus (DRN), where serotonergic neurons are most represented, and also in the hippocampus and amygdala [8]. In addition, TAAR1 expression was found predominantly in pyramidal neurons in the cortex layer V [5], confirming earlier evidence for the TAAR1 mRNA presence in the mouse brain frontal regions [9].
In animal models [10] and in preclinical studies [11], TAAR1 agonists were found to have procognitive [7], antipsychotic and antidepressant activity [7], which allows us to consider TAAR1 as a therapeutic target for the treatment of mental disorders [2, 12].
The experiments in vivo have shown that TAAR1 agonists reduces hyperlocomotion in pharmacological tests with cocaine and in dopamine transporter knockout models of hyperdopaminergy, and also reduces the action potential frequency in dopaminergic neurons [3, 7]. The evidence of hyposensitivity to amphetamine and constitutive activity of monoaminergic nuclei was found in a knockout mouse model with overexpression of TAAR1 in the brain [13]. In addition, the selective activation of TAAR1 by both partial and full agonists reduced the impulsive behavior in C57Bl/6J mice and NMDA receptor knockout mice [3, 5, 7, 13].
Although the TA functional significance remains unclear, it has been found that the changes in TA concentration and/or receptor dysfunction may be involved in a number of neuropsychiatric disorders associated with monoaminergic dysfunction, including schizophrenia, recurrent depressive disorder, Parkinson’s disease, attention deficit hyperactivity disorder [2, 14]. It was proved that TAAR1 knockout mice had similar behavioral changes to those observed in schizophrenia: an increase in locomotor activity with amphetamine use and a decrease in stereotypic reactions caused by apomorphine [4], an increase in the density of dopamine D2 receptors in the striatum [15], as well as a deficit in prepulse inhibition of the startle response [16]. TAAR1 receptor knockout mice demonstrated the synaptic function dysregulation mediated by the NMDA receptor in the prefrontal cortex expressed in stereotypical and impulsive behavior [5].
Sensory gating, along with mismatch negativity (MMN) and prepulse inhibition (PPI), is a recognized neurophysiological marker of schizophrenia [17]. Sensory gating is the process of dosing and filtering the environment information, with the help of which the brain regulates the magnitude of responses to sensory stimuli [18]. The standard paradigm consists of short sound stimuli with a fixed interstimulus interval within a pair of stimuli and a large interval between pairs of stimuli [19].
It was previously studied [20] that the TAAR1 agonist (RO 5263397) at a dosage of 1 mg/kg improves SG due to an increase in the N40 response amplitude to the first stimulus in the pair (S1), compared to the second stimulus in the pair (S2).
Since TAAR1 receptor agonists have a significant effect on SG parameters, it was decided to study the SG change in TAAR1 receptor of knockout mice.
MATERIALS AND METHODS
Animals. The study was conducted on 3–5-month-old male TAAR1 knockout mice (KO) (n = 11). The wild type (WT) males mice (n = 11) were used as controls. The average weight of the animals was 28–30g. The initial lines for WT and KO were 129S1/Sv and C57BL/6. The animals were obtained from the vivarium of the Institute of Translational Biomedicine (St. Petersburg State University, St. Petersburg, Russia). All animals were kept under standard conditions with ad libitum access to food and water and a 12-h light–dark cycle. Animals were housed in single boxes (30 cm × 15 cm × 17 cm). Prior to the start of all manipulations with animals, acclimatization was 7 days. All experiments were carried out in accordance with international standards for biomedical research with animals (European Convention for the Protection of Vertebrate Animals Used for Experimentation and other Scientific Purposes, 1986). The experimental protocol was approved by the Ethics Committee of the Faculty of Biology, St. Petersburg State University.
Surgery. Zoletil (70 mg/kg IM) and Xylazine (0.2 mg/kg IM) were used for anesthesia. Recording electrodes were implanted bilaterally (–6 mm posterior, 3.5 mm lateral to the bregma); the reference electrode was placed in the right hemisphere (–2.5 mm posterior, 3.5 mm lateral to the bregma); the ground electrode was placed in the left hemisphere (–2.5 mm posterior, 3.5 mm lateral to the bregma). Epidural electrodes were fixed with Acrodent cold polymerization dental plastic (AT Stoma, Ukraine). The operating field was treated with Baneocin® powder. Intramuscular injections of Bicillin-5 (benzathine benzylpenicillin, 100 mg/kg IM) were given at the end of the surgery. ERP recordings were conducted no earlier than 5 days after the surgery.
Experimental procedure. The experiment was carried out on chronic freely moving animals. Animals were housed in experimental plexiglass boxes (30 cm × 15 cm × 17 cm), and sounds was presented through loudspeakers located near the boxes. The experimental protocol included two identical auditory stimuli (S1 and S2) with a 10 ms duration and 3000 Hz frequency. Stimuli were presented at intensity of 85 dB. The interval between stimuli in a pair was 300 ms, the interval between pairs of stimuli varied within 3–5 s, 100 pairs were presented in each block. EEG registration was performed by a Mitsar-EEG-05/70-201 digital electroencephalograph (Mitsar Inc., St. Petersburg, Russia). Sounds were generated and presented by Psytask v.2.4 software (Mitsar Inc., St. Petersburg, Russia). EEG were recorded and processed by WinEEG v.2.4 software (Mitsar Inc., St. Petersburg, Russia). Processing included the removal of grooming artifacts (frequency 100–200 Hz; amplitude greater than ± 500 µV on any channel) [21]. The EEG signal was filtered in the 10–100 Hz band. Such filtering was chosen for the best detection of the P50/N40 component against the background of higher amplitude waves [22]. The N40 component in rodents is an analogue of the P50 component, which is commonly used to study SG in humans [23]. The amplitude of N40 was measured as an average over a period of 20–40 ms from the start of stimulus presentation. The SG index was calculated by (S1–S2) difference and (S2/S1) ratio [24].
Statistical analysis. All data had a normal distribution according to the Kolmogorov-Smirnov test. Statistical evaluation of group ERPs was carried out by repeated measures 2-way ANOVA with within-subjects factor “stimulus” (S1 and S2) and between-subjects factor “group” (WT and TAAR1-KO). If the analysis revealed a significant effect or an interaction, a post hoc analysis was performed by a paired Student’s t-test.
RESULTS
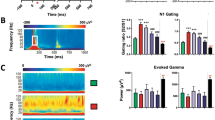
The data obtained show that the N40 amplitude in response to both the first (S1) and the second (S2) stimulus in the pair is significantly lower in TAAR1 knockout mice. The ERPs for TAAR1 knockout mice compared with wild-type mice (control group) in SG paradigm are shown in Fig. 1. Two-way ANOVA shows significant main effects of “group” (TAAR1_KO and WT; F(1, 10) = 11.282, p = 0.007) and “stimulus” (S1 and S2; F(1, 10) = 21.016, р ˂ 0.001) factors (Fig. 2). A significant “group” × “stimulus” interaction has been also revealed (F(1, 10) = 7.284, p = 0.022). Further analysis of the N40 amplitude obtained in response to S1 and S2 reveals a significant difference in S1 (p = 0.009) and S2 (p = 0.020) amplitude in TAAR1-KO and WT animals.
Figure 3a shows the SG index calculated by (S1–S2) difference. The graph shows that both the amplitude of the N40 component and the SG index are higher in wild-type mice compared to genetically modified animals (F(1, 10) = 7.284, p = 0.02). When comparing the SG index calculated by (S2/S1) ratio, no significant differences were found between groups of animals (F(1, 10) = 0.080, p = 0.783) (Fig. 3b).
A significant decrease in the N40 amplitude has been found in TAAR1-KO mice compared with wild-type mice. In addition, SG absolute value calculated by the (S1–S2) difference has been also reduced, but SG relative value calculated by the (S1/S2) ratio remains unchanged.
DISCUSSION
The results demonstrate a significant N40 attenuation in the TAAR1 knockout mice compared to the wild-type animals. The N40 amplitude decreases in response to both the first (S1) and the second (S2) stimuli in the pair. Accordingly, this leads to a decrease in the absolute values of sensory gating in the TAAR1-KO group. However, the SG index, which shows the relative response magnitude suppression to the second stimulus, remains unchanged.
Previously, in chronic experiments on awake C57BL/6 mice, it has been found that the TAAR1 receptor agonist RO5263397 administration at a dose of 1 mg/kg significantly improves the sensory gating index calculated by the (S1–S2) difference [20]. Moreover, the increase in the SG index occurred due to an increase in the N40 amplitude of the auditory ERP component in response to the first stimulus (S1) in the pair. Thus, the TAAR1 stimulation leads to an increase in the N40 amplitude, and the absence of receptors is accompanied by a significant N40 decrease.
Sensory gating, along with mismatch negativity (MMN) and prepulse inhibition (PPI), is a widely used neurophysiological biomarker of schizophrenia [17]. N40 sensory gating in mice is considered to be analogous to P50 sensory gating in humans. Interestingly, according to a number of studies, P50 SG violation in schizophrenia is mainly associated with a decreased amplitude in response to the first stimulus in a pair [18]. Our data indicate a possible TAAR1 involvement in SG disturbances in schizophrenia. It should be noted that the SG data is far from homogeneous. Along with this, there is an evidence that the SG coefficient does not depend on the amplitude of the first stimulus in the pair (S1) [26], and the classic experimental works of Freedman [19, 27, 28] associated a low SG with a poor ability to suppress the second stimulus in the pair (S2).
A suppression of the second stimulus response, on the one hand, is presumably associated with the inhibitory mechanisms underlying SG. At the same time, the decrease in amplitude may be due to an increase in the neuronal populations refractoriness that generate the corresponding ERP components. Since this work revealed a noticeable decrease in the N40 electrogenesis, it is still premature to make a final conclusion about the nature of the change in SG itself upon elimination of TAAR1 receptors.
The present study showed that TAAR1 play an important role in the mechanisms of auditory ERP generation, since the N40 amplitude of auditory ERPs significantly decreases in genetically modified animals. A study of the role of the system of trace amines and TAAR1 in the sensory dosing and filtering is of interest for understanding the pathogenesis of neuropsychiatric disorders.
REFERENCES
Millan MJ, Rivet JM, Gobert A (2016) The frontal cortex as a network hub controlling mood and cognition: Probing its neurochemical substrates for improved therapy of psychiatric and neurological disorders. J Psychopharmacol 30: 1099–1128. https://doi.org/10.1177/0269881116672342
Berry MD, Gainetdinov RR, Hoener MC, Shahid M (2017) Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges. Pharmacol Ther 180: 161–180. https://doi.org/10.1016/j.pharmthera.2017.07.002
Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC (2011) TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci U S A 108: 8485–8490. https://doi.org/10.1073/pnas.1103029108
Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL, Hoener MC (2008) Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther 324: 948–956. https://doi.org/10.1124/jpet.107.132647
Espinoza S, Lignani G, Caffino L, Maggi S, Sukhanov I, Leo D, Mus L, Emanuele M, Ronzitti G, Harmeier A, Medrihan L, Sotnikova TD, Chieregatti E, Hoener MC, Benfenati F, Tucci V, Fumagalli F, Gainetdinov RR (2015) TAAR1 Modulates Cortical Glutamate NMDA Receptor Function. Neuropsychopharmacology 40: 2217–2227. https://doi.org/10.1038/npp.2015.65
Sukhanov I, Caffino L, Efimova EV, Espinoza S, Sotnikova TD, Cervo L, Fumagalli F, Gainetdinov RR (2016) Increased context-dependent conditioning to amphetamine in mice lacking TAAR1. Pharmacol Res 103: 206–214. https://doi.org/10.1016/j.phrs.2015.11.002
Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, Buchy D, Metzler V, Chaboz S, Groebke Zbinden K, Galley G, Norcross RD, Tuerck D, Bruns A, Morairty SR, Kilduff TS, Wallace TL, Risterucci C, Wettstein JG, Hoener MC (2013) A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry 18: 543–556. https://doi.org/10.1038/mp.2012.57
John J, Kukshal P, Bhatia T, Chowdari KV, Nimgaonkar VL, Deshpande SN, Thelma BK (2017) Possible role of rare variants in Trace amine associated receptor 1 in schizophrenia. Schizophr Res 189: 190–195. https://doi.org/10.1016/j.schres.2017.02.020
di Cara B, Maggio R, Aloisi G, Rivet JM, Lundius EG, Yoshitake T, Svenningsson P, Brocco M, Gobert A, de Groote L, Cistarelli L, Veiga S, de Montrion CD, Rodriguez M, Galizzi JP, Lockhart BP, Cogé F, Boutin JA, Vayer P, Verdouw PM, Groenink L, Millan MJ (2011) Genetic deletion of trace amine 1 receptors reveals their role in auto-inhibiting the actions of ecstasy (MDMA). J Neurosci 31: 16928–16940. https://doi.org/10.1523/JNEUROSCI.2502-11.2011
Dedic N, Jones PG, Hopkins SC, Lew R, Shao L, Campbell JE, Spear KL, Large TH, Campbell UC, Hanania T, Leahy E, Koblan KS (2019) SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J Pharmacol Exp Ther 371: 1–14. https://doi.org/10.1124/jpet.119.260281
Koblan KS, Kent J, Hopkins SC, Krystal JH, Cheng H, Goldman R, Loebel A (2020) A Non–D2-Receptor-Binding Drug for the Treatment of Schizophrenia. N Engl J Med 382: 1497–1506. https://doi.org/10.1056/nejmoa1911772
Gainetdinov RR, Hoener MC, Berry MD (2018) Trace amines and their receptors. Pharmacol Rev 70: 549–620. https://doi.org/10.1124/pr.117.015305
Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, André CB, Haenggi M, Miss MT, Galley G, Norcross RD, Invernizzi RW, Wettstein JG, Moreau JL, Hoener MC (2012) Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine. Neuropsychopharmacology 37: 2580–2592. https://doi.org/10.1038/npp.2012.109
Rutigliano G, Accorroni A, Zucchi R (2018) The case for TAAR1 as a modulator of central nervous system function. Front Pharmacol 8: 987. https://doi.org/10.3389/fphar.2017.00987
Corripio I, Escartí MJ, Portella MJ, Pérez V, Grasa E, Sauras RB, Alonso A, Safont G, Camacho MV, Dueñas R, Arranz B, San L, Catafau AM, Carrió I, Álvarez E (2011) Density of striatal D2 receptors in untreated first-episode psychosis: an I 123-IBZM SPECT study. Eur Neuropsychopharmacol 21: 861–866. https://doi.org/10.1016/j.euroneuro.2011.03.004
Polyakova NV, Vinogradova EP, Aleksandrov AA, Gainetdinov RR (2018) Prepulse inhibition in the TAAR1 knockout mice. Rossiiskii fiziologicheskii zhurnal 104: 1098–1105.
Xia L, Wang D, Wang J, Xu H, Huo L, Tian Y, Dai Q, Wei S, Wang W, Zhang G, Du X, Jia Q, Zhu X, Wang L, Tang W, Zhang XY (2020) Association of cognitive and P50 suppression deficits in chronic patients with schizophrenia. Clin Neurophysiol 131: 725–733. https://doi.org/10.1016/j.clinph.2019.12.405
Javitt DC, Freedman R (2015) Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry 172: 17–31. https://doi.org/10.1176/appi.ajp.2014.13121691
Freedman R, Waldo M, Bickford-Wimer P, Nagamoto H (1991) Elementary neuronal dysfunctions in schizophrenia. Schizophr Res 4: 233–243. https://doi.org/10.1016/0920-9964(91)90035-P
Aleksandrov AA, Dmitrieva ES, Volnova AB, Knyazeva VM, Polyakova NV, Ptukha MA, Gainetdinov RR (2019) Effect of alpha-NETA on auditory event related potentials in sensory gating study paradigm in mice. Neurosci Lett 712: 134470. https://doi.org/10.1016/j.neulet.2019.134470
Roger C, Hasbroucq T, Rabat A, Vidal F, Burle B (2009) Neurophysics of temporal discrimination in the rat: A mismatch negativity study. Psychophysiology 46: 1028–1032. https://doi.org/10.1111/j.1469-8986.2009.00840.x
Rentzsch J, Jockers-Scherübl MC, Boutros NN, Gallinat J (2008) Test-retest reliability of P50, N100 and P200 auditory sensory gating in healthy subjects. Int J Psychophysiol 67: 81–90. https://doi.org/10.1016/j.ijpsycho.2007.10.006
Mears RP, Klein AC, Cromwell HC (2006) Auditory inhibitory gating in medial pre-frontal cortex: Single unit and local field potential analysis. Neuroscience 141 (1): 47–65.
Boutros NN, Korzyukov O, Jansen B, Feingold A, Bell M (2004) Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Res 126: 203–215. https://doi.org/10.1016/j.psychres.2004.01.007
Karkal R, Goyal N, Tikka SK, Khanande RV, Kakunje A, Khess CRJ (2018) Sensory gating deficits and their clinical correlates in drug-free/drug-naive patients with schizophrenia. Indian J Psychol Med 40: 247–256. https://doi.org/10.4103/IJPSYM.IJPSYM_53_18
Clementz BA, Blumenfeld LD, Cobb S (1997) The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport 8: 3889–3893. https://doi.org/10.1097/00001756-199712220-00010
Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD (1983) Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: Comparison of medicated and drug-free patients. Biol Psychiatry 18(5): 537–551.
Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, Drebing C, Nagamoto H, Bickford-Wimer P, Franks R (1987) Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull 13(4): 669–678.
Funding
This work was supported by the Russian Science Foundation (RSF), project no. 22-25-00006.
Author information
Authors and Affiliations
Contributions
AAA provided the concept of the study; AAA, VMK, ESD, and YAS provided experimental design; ESD, VMK, YAS contributed to data acquisition and analysis. All authors equally contributed to the interpretation and discussion of the results, writing and critical revision of the manuscript.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have neither evident not potential conflict of interest related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2022, Vol. 58, No. 4, pp. 291–297https://doi.org/10.31857/S0044452922040027.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aleksandrov, A.A., Dmitrieva, E.S., Knyazeva, V.M. et al. Sensory Gating in TAAR1 Knockout Mice. J Evol Biochem Phys 58, 979–985 (2022). https://doi.org/10.1134/S0022093022040044
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022040044