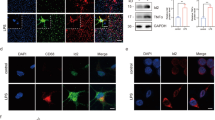
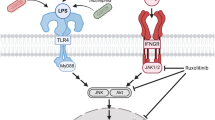
Abstract
Neurodegenerative diseases are accompanied by neuroinflammation and excitotoxicity, in which microglia and astrocytes play an important role. However, there is still no clear understanding of how glial cells interact in the processes associated with inflammatory reactions in the brain. The study was aimed to elucidate the in vitro effect of bacterial lipopolysaccharide (LPS), as an inducer of inflammatory reactions, on transcription levels of the Slc superfamily transporter genes in microglia, depending on the astrocytic environment, as well as to reveal regional differences in these levels. The study was carried out on microglial cells in both monoculture and co-culture with astrocytes. Microglia and astrocytes were isolated from the cortex and hippocampus of 21-day-old Wistar rats. Microglial activation was induced by LPS. Transcription levels of the genes encoding Slc family transporters were analyzed by reverse transcription polymerase chain reaction (RT-PCR). LPS was shown to induce an increase in transcription levels of genes encoding the neuronal excitatory amino acid transporter 3 (Slc1a1, EAAT3), excitatory amino acid transporter 4 (Slc1a6, EAAT4), vesicular glutamate transporter 1 (Slc17a7, VGLUT1), and monocarboxylate transporter 1 (Slc16a1, MCT1a1) in microglial cells in vitro both in monoculture and in the presence of astrocytes. Transcription levels of the Slc family transporter genes significantly increased in all studied cultures compared to non-activated microglial cultures (control): in non-activated microglia-astrocyte co-cultures by 30.8–172.3%, in LPS-activated microglial monocultures by 33.5–63.3%, in LPS-activated microglia-astrocyte co-cultures by 17.8–76.0%. Moreover, regional differences were revealed in transcription levels of the above transporter genes in response to LPS administration. Thus, the data obtained confirm a change in the transcription profile of glutamate and monocarboxylate transporters upon LPS-induced activation of microglia, as well as the involvement of the astrocytic environment in these changes. Overall, it was shown that the exposure to a proinflammatory factor (LPS) activates the transcription of Slc family transporter genes in microglial cells.



Similar content being viewed by others
REFERENCES
Deuschl G, Beghi E, Fazekas F, Varga T, Christoforidi KA, Sipido E, Bassetti CL, Vos T, Feigin VL (2020) The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. The Lancet Public Health 5:e551–e567. https://doi.org/10.1016/S2468-2667(20)30190-0
Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, Boza-Serrano A (2018) Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front Cell Neurosci 12:488. https://doi.org/10.3389/FNCEL.2018.00488/FULL
Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, de Witte P, Ballini C, Corte L della, Dexter D (2009) Neuro-inflammation induced in the hippocampus of “binge drinking” rats may be mediated by elevated extracellular glutamate content. J Neurochem 111:1119–1128. https://doi.org/10.1111/J.1471-4159.2009.06389.X
Takaki J, Fujimori K, Miura M, Suzuki T, Sekino Y, Sato K (2012) L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: The “collusion” hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J Neuroinflammat 9:1–17. https://doi.org/10.1186/1742-2094-9-275/FIGURES/9
O’Donovan SM, Sullivan CR, McCullumsmith RE (2017) The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ Schizophr 2017 3:1 3:1–14. https://doi.org/10.1038/s41537-017-0037-1
Persson M, Rönnbäck L (2011) Microglial self-defence mediated through GLT-1 and glutathione. Amino Acids 2011 42:1 42:207–219. https://doi.org/10.1007/S00726-011-0865-7
Rodríguez A, Ortega A (2017) Glutamine/Glutamate Transporters in Glial Cells: Much More Than Participants of a Metabolic Shuttle. Adv Neurobiol 16:169–183. https://doi.org/10.1007/978-3-319-55769-4_8
Pajarillo E, Rizor A, Lee J, Aschner M, Lee E (2019) The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 161:107559. https://doi.org/10.1016/J.NEUROPHARM.2019.03.002
Liang J, Takeuchi H, Doi Y, Kawanokuchi J, Sonobe Y, Jin S, Yawata I, Li H, Yasuoka S, Mizuno T, Suzumura A (2008) Excitatory amino acid transporter expression by astrocytes is neuroprotective against microglial excitotoxicity. Brain Res 1210:11–19. https://doi.org/10.1016/j.brainres.2008.03.012
Ding L, Xu X, Li C, Wang Y, Xia X, Zheng JC (2021) Glutaminase in microglia: A novel regulator of neuroinflammation. Brain Behav Immun 92:139–156. https://doi.org/10.1016/J.BBI.2020.11.038
Mason S (2017) Lactate shuttles in neuroenergetics-homeostasis, allostasis and beyond. Front Neurosci 11:43. https://doi.org/10.3389/FNINS.2017.00043
Pérez-Escuredo J, van Hée VF, Sboarina M, Falces J, Payen VL, Pellerin L, Sonveaux P (2016) Monocarboxylate transporters in the brain and in cancer. Biochim Biophys Acta 1863:2481. https://doi.org/10.1016/J.BBAMCR.2016.03.013
He JH, Yu L, Wang ZY, Wang Q, Cao JL, Gu LB (2019) Inhibition Of Monocarboxylate Transporter 1 In Spinal Cord Horn Significantly Reverses Chronic Inflammatory Pain. J Pain Res 12:2981. https://doi.org/10.2147/JPR.S219359
Kong L, Wang Z, Liang X, Wang Y, Gao L, Ma C (2019) Monocarboxylate transporter 1 promotes classical microglial activation and pro-inflammatory effect via 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3. J Neuroinflammation 16:240. https://doi.org/10.1186/S12974-019-1648-4
Moreira TJTP, Pierre K, Maekawa F, Repond C, Cebere A, Liljequist S, Pellerin L (2009) Enhanced cerebral expression of MCT1 and MCT2 in a rat ischemia model occurs in activated microglial cells. J Cereb Blood Flow Metab 29:1273–1283. https://doi.org/10.1038/JCBFM.2009.50
Cabinio M, Saresella M, Piancone F, LaRosa F, Marventano I, Guerini FR, Nemni R, Baglio F, Clerici M (2018) Association between Hippocampal Shape, Neuroinflammation, and Cognitive Decline in Alzheimer’s Disease. J Alzheimers Dis 66:1131–1144. https://doi.org/10.3233/JAD-180250
Harrison NA, Cercignani M, Voon V, Critchley HD (2015) Effects of Inflammation on Hippocampus and Substantia Nigra Responses to Novelty in Healthy Human Participants. Neuropsychopharmacology 40:831. https://doi.org/10.1038/NPP.2014.222
de Pablos RM, Villarán RF, Argüelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A (2006) Stress Increases Vulnerability to Inflammation in the Rat Prefrontal Cortex. J Neurosci 26:5709. https://doi.org/10.1523/JNEUROSCI.0802-06.2006
Antunes MM, Godoy G, Masi LN, Curi R, Bazotte RB (2021) Prefrontal Cortex and Hippocampus Inflammation in Mice Fed High-Carbohydrate or High-Fat Diets. J Med Food https://home.liebertpub.com/jmf. https://doi.org/10.1089/JMF.2021.0026
Grabert K, Michoel T, Karavolos MH, Clohisey S, Kenneth Baillie J, Stevens MP, Freeman TC, Summers KM, McColl BW (2016) Microglial brain region-dependent diversity and selective regional sensitivities to ageing. Nat Neurosci 19:504. https://doi.org/10.1038/NN.4222
Mecha M (2011) An easy and fast way to obtain a high number of glial cells from rat cerebral tissue: A beginners approach. Protoc Exch. https://doi.org/10.1038/PROTEX.2011.218
Collins HY, Bohlen CJ (2018) Isolation and culture of rodent microglia to promote a dynamic ramified morphology in serum-free medium. J Vis Exp 9 (133):57122. https://doi.org/10.3791/57122
Bohlen CJ, Bennett FC, Tucker AF, Collins HY, Mulinyawe SB, Barres BA (2017) Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 94:759-773. https://doi.org/10.1016/J.NEURON.2017.04.043
Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA, Giger RJ, Stevens B (2016) New tools for studying microglia in the mouse and human CNS. Proc Nat Acad Sci USA 113:E1738–E1746. https://doi.org/10.1073/pnas.1525528113
Taticchi A, Urbani S, Albi E, Servili M, Codini M, Traina G, Balloni S, Patria FF, Perioli L, Beccari T, Conte C (2019) In Vitro Anti-Inflammatory Effects of Phenolic Compounds from Moraiolo Virgin Olive Oil (MVOO) in Brain Cells via Regulating the TLR4/NLRP3 Axis. Molecules 10 (24):453. https://doi.org/10.3390/MOLECULES24244523
Tarassishin L, Suh HS, Lee SC (2014) LPS and IL-1 differentially activate mouse and human astrocytes: Role of CD14. GLIA 62:999–1013. https://doi.org/10.1002/glia.22657
Moura AC de, Lazzari VM, Agnes G, Almeida S, Giovenardi M, Veiga AB eatriz G da (2014) Transcriptional expression study in the central nervous system of rats: what gene should be used as internal control? Einstein (Sao Paulo) 12:336–341. https://doi.org/10.1590/S1679-45082014AO3042
Montiel T, Camacho A, Estrada-Sanchez AM, Massieu L (2005) Differential effects of the substrate inhibitor l-trans-pyrrolidine-2,4-dicarboxylate (PDC) and the non-substrate inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) of glutamate transporters on neuronal damage and extracellular amino acid levels in rat brain in vivo. Neuroscience 133:667–678. https://doi.org/10.1016/J.NEUROSCIENCE.2004.11.020
Rosa JM, Farré-Alins V, Ortega MC, Navarrete M, Lopez-Rodriguez AB, Palomino-Antolín A, Fernández-López E, Sol VV, Decouty C, Narros-Fernández P, Clemente D, Egea J (2021) TLR4 pathway impairs synaptic number and cerebrovascular functions through astrocyte activation following traumatic brain injury. Br J Pharmacol 178:3395–3413. https://doi.org/10.1111/BPH.15488
Zou JY, Crews FT (2005) TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res 1034:11–24. https://doi.org/10.1016/J.BRAINRES.2004.11.014
Gras G, Samah B, Hubert A, Léone C, Porcheray F, Rimaniol AC (2012) EAAT expression by macrophages and microglia: still more questions than answers. Amino Acids 42:221–229. https://doi.org/10.1007/S00726-011-0866-6
Krishnan VS, Shavlakadze T, Grounds MD, Hodgetts SI, Harvey AR (2018) Age-related loss of VGLUT1 excitatory, but not VGAT inhibitory, immunoreactive terminals on motor neurons in spinal cords of old sarcopenic male mice. Biogerontology 19:385–399. https://doi.org/10.1007/S10522-018-9765-5
Draoui N, Feron O (2011) Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech 4:727–732. https://doi.org/10.1242/DMM.007724
Jha MK, Morrison BM (2020) Lactate Transporters Mediate Glia-Neuron Metabolic Crosstalk in Homeostasis and Disease. Front Cell Neurosci 14:589582. https://doi.org/10.3389/FNCEL.2020.589582/FULL
López-Redondo F, Nakajima K, Honda S, Kohsaka S (2000) Glutamate transporter GLT-1 is highly expressed in activated microglia following facial nerve axotomy. Brain Res Mol Brain Res 76:429–435. https://doi.org/10.1016/S0169-328X(00)00022-X
Rönnbäck L, Hansson E (2004) On the potential role of glutamate transport in metal fatigue. J Neuroinflammat 1:1–9. https://doi.org/10.1186/1742-2094-1-22/FIGURES/1
Tilleux S, Hermans E (2007) Neuroinflammation and Regulation of Glial Glutamate Uptake in Neurological Disorders. J Neurosci Res 85:2059-2070. https://doi.org/10.1002/jnr.21325
Du X, Li J, Li M, Yang X, Qi Z, Xu B, Liu W, Xu Z, Deng Y (2020) Research progress on the role of type I vesicular glutamate transporter (VGLUT1) in nervous system diseases. Cell Biosci 10:1–10. https://doi.org/10.1186/S13578-020-00393-4/METRICS
Leiguarda C, McCarthy CJ, Casadei M, Lundgren KH, Coronel MF, Trigosso-Venario H, Seal RP, Seroogy KB, Brumovsky PR (2020) Transcript Expression of Vesicular Glutamate Transporters in Rat Dorsal Root Ganglion and Spinal Cord Neurons: Impact of Spinal Blockade during Hindpaw Inflammation. ACS Chem Neurosci 11:2602–2614. https://doi.org/10.1021/ACSCHEMNEURO.0C00272
Brioschi S, d’Errico P, Amann LS, Janova H, Wojcik SM, Meyer-Luehmann M, Rajendran L, Wieghofer P, Paolicelli RC, Biber K (2020) Detection of Synaptic Proteins in Microglia by Flow Cytometry. Front Mol Neurosci 13:149. https://doi.org/10.3389/FNMOL.2020.00149/BIBTEX
Marín-Teva JL, Cuadros MA, Martín-Oliva D, Navascués J (2011) Microglia and neuronal cell death. Neuron Glia Biol 7:25–40. https://doi.org/10.1017/S1740925X12000014
Graeber MB (2010) Changing face of microglia. Science (New York, NY) 330:783–788. https://doi.org/10.1126/SCIENCE.1190929
Malik AR, Willnow TE (2019) Excitatory Amino Acid Transporters in Physiology and Disorders of the Central Nervous System. Int J Mol Sci 20:5671. https://doi.org/10.3390/IJMS20225671
Liu C, Zhang Y, Liu Q, Jiang L, Li M, Wang S, Long T, He W, Kong X, Qin G, Chen L, Zhang Y, Zhou J (2018) P2X4-receptor participates in EAAT3 regulation via BDNF-TrkB signaling in a model of trigeminal allodynia. Mol Pain 14:1–15. https://doi.org/10.1177/1744806918795930
Liang J, Chao D, Sandhu HK, Yu Y, Zhang L, Balboni G, Kim DH, Xia Y (2014) δ-Opioid receptors up-regulate excitatory amino acid transporters in mouse astrocytes. Br J Pharmacol 171:5417–5430. https://doi.org/10.1111/BPH.12857
Massie A, Cnops L, Smolders I, McCullumsmith R, Kooijman R, Kwak S, Arckens L, Michotte Y (2008) High-affinity Na+/K+-dependent glutamate transporter EAAT4 is expressed throughout the rat fore- and midbrain. J Comp Neurol 511:155–172. https://doi.org/10.1002/CNE.21823
Ciesielska A, Matyjek M, Kwiatkowska K (2021) TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci: CMLS 78:1233–1261. https://doi.org/10.1007/S00018-020-03656-Y
O’Callaghan P, Li JP, Lannfelt L, Lindahl U, Zhang X (2015) Microglial Heparan Sulfate Proteoglycans Facilitate the Cluster-of-Differentiation 14 (CD14)/Toll-like Receptor 4 (TLR4)-Dependent Inflammatory Response. J Biol Chem 290:14904–14914. https://doi.org/10.1074/JBC.M114.634337
Korn T, Magnus T, Jung S (2005) Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: a mechanism mediated by tumor necrosis factor-alpha. FASEB J 19:1878–1880. https://doi.org/10.1096/FJ.05-3748FJE
Haroon E, Miller AH, Sanacora G (2016) Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 42:193–215. https://doi.org/10.1038/npp.2016.199
Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P (2013) The Cystine/Glutamate Antiporter System xc– in Health and Disease: From Molecular Mechanisms to Novel Therapeutic Opportunities. Antioxid Redox Signal 18:522. https://doi.org/10.1089/ARS.2011.4391
Kitagawa Y, Nakaso K, Horikoshi Y, Morimoto M, Omotani T, Otsuki A, Inagaki Y, Sato H, Matsura T (2019) System xc– in microglia is a novel therapeutic target for post-septic neurological and psychiatric illness. Sci Rep 9:1–13. https://doi.org/10.1038/s41598-019-44006-8
Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J (2016) Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun 7:12540. https://doi.org/10.1038/NCOMMS12540
Filipello F, Morini R, Corradini I, Zerbi V, Canzi A, Michalski B, Erreni M, Markicevic M, Starvaggi-Cucuzza C, Otero K, Piccio L, Cignarella F, Perrucci F, Tamborini M, Genua M, Rajendran L, Menna E, Vetrano S, Fahnestock M, Paolicelli RC, Matteoli M (2018) The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity 48:979–991.e8. https://doi.org/10.1016/J.IMMUNI.2018.04.016
Tanaka T, Murakami K, Bando Y, Yoshida S (2015) Interferon regulatory factor 7 participates in the M1-like microglial polarization switch. Glia 63:595–610. https://doi.org/10.1002/GLIA.22770
Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, Ffrench-Constant C (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16:1211–1218. https://doi.org/10.1038/NN.3469
Kelly B, O’Neill LAJ (2015) Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 25:771–784. https://doi.org/10.1038/CR.2015.68
Morgun AV, Malinovskaja NA, Komleva JuK, Lopatina OL, Kuvacheva NV, Panina JuA, Taranushenko TE, Solonchuk RJu, Salmina AB (2014) Structural and functional Heterogenety of astrocytes in the brain: role in neurodegeneration and neuroinflammation. Bull Sib Med 13: 138-148. https://doi.org/10.20538/1682-0363-2014-5-138-148
Anderson MA, Ao Y, Sofroniew MV (2014) Heterogeneity of reactive astrocytes. Neurosci Lett 565:23–29. https://doi.org/10.1016/J.NEULET.2013.12.030
Matias I, Morgado J, Gomes FCA (2019) Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front Aging Neurosci 11:59. https://doi.org/10.3389/FNAGI.2019.00059/BIBTEX
Tan YL, Yuan Y, Tian L (2019) Microglial regional heterogeneity and its role in the brain. Mol Psychiatry 25:351–367. https://doi.org/10.1038/s41380-019-0609-8
Furube E, Kawai S, Inagaki H, Takagi S, Miyata S (2018) Brain Region-dependent Heterogeneity and Dose-dependent Difference in Transient Microglia Population Increase during Lipopolysaccharide-induced Inflammation. Sci Rep 8:2203. https://doi.org/10.1038/S41598-018-20643-3
Doorn KJ, Brevé JJP, Drukarch B, Boddeke HW, Huitinga I, Lucassen PJ, van Dam AM (2015) Brain region-specific gene expression profiles in freshly isolated rat microglia. Front Cell Neurosci 9:84. https://doi.org/10.3389/FNCEL.2015.00084
Stratoulias V, Venero JL, Tremblay M-È, Joseph B (2019) Microglial subtypes: diversity within the microglial community. EMBO J 38:e101997. https://doi.org/10.15252/EMBJ.2019101997
Kreisel T, Wolf B, Keshet E, Licht T (2019) Unique role for dentate gyrus microglia in neuroblast survival and in VEGF-induced activation. Glia 67:594–618. https://doi.org/10.1002/GLIA.23505
Bisht K, Sharma KP, Lecours C, Gabriela Sánchez M, el Hajj H, Milior G, Olmos-Alonso A, Gómez-Nicola D, Luheshi G, Vallières L, Branchi I, Maggi L, Limatola C, Butovsky O, Tremblay MÈ (2016) Dark microglia: A new phenotype predominantly associated with pathological states. Glia 64:826–839. https://doi.org/10.1002/GLIA.22966
Funding
This work was supported by the Russian Foundation for Basic Research (RFBR), grant no. 18-34-00152.
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design (I.N.D.); data collection (I.A.S.); data processing (I.A.S., I.N.D.); writing and editing the manuscript (I.A.S., I.N.D.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest, both evident and potential, associated with the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 3, pp. 379–396https://doi.org/10.31857/S0869813922030098.
Rights and permissions
About this article
Cite this article
Starovoytova, I.A., Dominova, I.N. An in vitro Study of the Effect of Bacterial Lipopolysaccharide on Transcription Levels of SLC Family Transporter Genes in Microglia. J Evol Biochem Phys 58, 508–522 (2022). https://doi.org/10.1134/S0022093022020193
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022020193