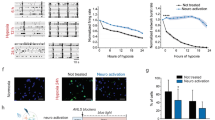
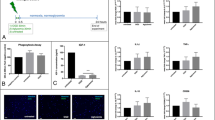
Abstract
Lactate has received attention as a potential therapeutic intervention for brain diseases, particularly those including energy deficit, exacerbated inflammation, and disrupted redox status, such as cerebral ischemia. However, lactate roles in metabolic or signaling pathways in neural cells remain elusive in the hypoxic and ischemic contexts. Here, we tested the effects of lactate on the survival of a microglial (BV-2) and a neuronal (SH-SY5Y) cell lines during oxygen and glucose deprivation (OGD) or OGD followed by reoxygenation (OGD/R). Lactate signaling was studied by using 3,5-DHBA, an exogenous agonist of lactate receptor GPR81. Inhibition of lactate dehydrogenase (LDH) or monocarboxylate transporters (MCT), using oxamate or 4-CIN, respectively, was performed to evaluate the impact of lactate metabolization and transport on cell viability. The OGD lasted 6 h and the reoxygenation lasted 24 h following OGD (OGD/R). Cell viability, extracellular lactate concentrations, microglial intracellular pH and TNF-ɑ release, and neurite elongation were evaluated. Lactate or 3,5-DHBA treatment during OGD increased microglial survival during reoxygenation. Inhibition of lactate metabolism and transport impaired microglial and neuronal viability. OGD led to intracellular acidification in BV-2 cells, and reoxygenation increased the release of TNF-ɑ, which was reverted by lactate and 3,5-DHBA treatment. Our results suggest that lactate plays a dual role in OGD, acting as a metabolic and a signaling molecule in BV-2 and SH-SY5Y cells. Lactate metabolism and transport are vital for cell survival during OGD. Moreover, lactate treatment and GPR81 activation during OGD promote long-term adaptations that potentially protect cells against secondary cell death during reoxygenation.
Graphical Abstract









Similar content being viewed by others
Data Availability
Data are available upon reasonable request.
Abbreviations
- 3,5-DHBA:
-
3,5-Dihydroxybenzoic acid
- 4-CIN:
-
ɑ-Cyano-4-hydroxycinnamic acid
- ANLS:
-
Astrocyte-neuron lactate shuttle
- ATP:
-
Adenosine triphosphate
- BCECF-AM:
-
Acetoxymethyl ester of 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein
- cAMP:
-
Cyclic adenosine monophosphate
- CD206:
-
Cluster of differentiation 206, mannose receptor
- CNS:
-
Central nervous system
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- ELISA:
-
Enzyme-linked immunosorbent assay
- FBS:
-
Fetal bovine serum
- GPR81:
-
G protein-coupled-receptor 81
- HCAR1:
-
Hydroxy-carboxylic acid receptor 1
- HCl:
-
Hydrochloric acid
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- HIF-1α:
-
Hypoxia inducible factor-1, subunit alpha
- OGD:
-
Oxygen and glucose deprivation
- OGD/R:
-
Oxygen and glucose deprivation-reoxygenation
- MCT:
-
Monocarboxylate transporter
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAD+ :
-
Nicotinamide adenine dinucleotide, oxidized form
- NADH:
-
Nicotinamide adenine dinucleotide, reduced form
- NEFL:
-
Neurofilament light polypeptide
- NF-κB:
-
Nuclear factor kappa B
- NLRP3:
-
Nod-like receptor protein 3
- pHi:
-
Intracellular potential of hydrogen
- P/S:
-
Penicillin/streptomycin
- PBS:
-
Phosphate-buffer saline
- PKA:
-
Protein kinase A
- RA:
-
Retinoic acid
- RT:
-
Room temperature
- TNF-α:
-
Tumor necrosis factor-alpha
References
Berthet C, Lei H, Thevenet J et al (2009) Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab 29:1780–1789. https://doi.org/10.1038/jcbfm.2009.97
Hamdy N, Eide S, Sun H-S, Feng Z-P (2020) Animal models for neonatal brain injury induced by hypoxic ischemic conditions in rodents. Exp Neurol 334:113457. https://doi.org/10.1016/j.expneurol.2020.113457
Tassinari IDÁ, Andrade MKG, da Rosa LA et al (2020) Lactate administration reduces brain injury and ameliorates behavioral outcomes following neonatal hypoxia–ischemia. Neuroscience 448:191–205. https://doi.org/10.1016/j.neuroscience.2020.09.006
Roumes H, Dumont U, Sanchez S et al (2021) Neuroprotective role of lactate in rat neonatal hypoxia-ischemia. J Cereb Blood Flow Metab 41:342–358. https://doi.org/10.1177/0271678X20908355
Azevedo PN, Zanirati G, Venturin GT et al (2020) Long-term changes in metabolic brain network drive memory impairments in rats following neonatal hypoxia-ischemia. Neurobiol Learn Mem 171:107207. https://doi.org/10.1016/j.nlm.2020.107207
Buscemi L, Blochet C, Magistretti PJ, Hirt L (2021) Hydroxycarboxylic acid receptor 1 and neuroprotection in a mouse model of cerebral ischemia-reperfusion. Front Physiol 12:689239. https://doi.org/10.3389/fphys.2021.689239
You Q, Lan X-B, Liu N et al (2023) Neuroprotective strategies for neonatal hypoxic-ischemic brain damage: current status and challenges. Eur J Pharmacol 957:176003. https://doi.org/10.1016/j.ejphar.2023.176003
Molloy EJ, Branagan A, Hurley T et al (2023) Neonatal encephalopathy and hypoxic-ischemic encephalopathy: moving from controversy to consensus definitions and subclassification. Pediatr Res. https://doi.org/10.1038/s41390-023-02775-z
Deng Q, Wu C, Liu TC-Y et al (2023) Exogenous lactate administration: a potential novel therapeutic approach for neonatal hypoxia-ischemia. Exp Neurol. https://doi.org/10.1016/j.expneurol.2023.114450
Szrejder M, Typiak M, Pikul P et al (2023) Role of l-lactate as an energy substrate in primary rat podocytes under physiological and glucose deprivation conditions. Eur J Cell Biol 102:151298. https://doi.org/10.1016/j.ejcb.2023.151298
Bouzat P, Oddo M (2014) Lactate and the injured brain: friend or foe? Curr Opin Crit Care 20:133–140. https://doi.org/10.1097/MCC.0000000000000072
Lev-Vachnish Y, Cadury S, Rotter-Maskowitz A et al (2019) L-Lactate promotes adult hippocampal neurogenesis. Front Neurosci 13:403. https://doi.org/10.3389/fnins.2019.00403
Kitaoka Y, Takahashi K, Hatta H (2022) Inhibition of monocarboxylate transporters (MCT) 1 and 4 reduces exercise capacity in mice. Physiol Rep 10:e15457. https://doi.org/10.14814/phy2.15457
Korol DL, Gardner RS, Tunur T, Gold PE (2019) Involvement of lactate transport in two object recognition tasks that require either the hippocampus or striatum. Behav Neurosci 133:176–187. https://doi.org/10.1037/bne0000304
Manosalva C, Quiroga J, Hidalgo AI et al (2021) Role of lactate in inflammatory processes: friend or foe. Front Immunol 12:808799. https://doi.org/10.3389/fimmu.2021.808799
Zhang M, Wang Y, Bai Y et al (2022) Monocarboxylate transporter 1 may benefit cerebral ischemia via facilitating lactate transport from glial cells to neurons. Front Neurol 13:781063. https://doi.org/10.3389/fneur.2022.781063
Kong L, Wang Z, Liang X et al (2019) Monocarboxylate transporter 1 promotes classical microglial activation and pro-inflammatory effect via 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3. J Neuroinflamm 16:240. https://doi.org/10.1186/s12974-019-1648-4
Dias C, Fernandes E, Barbosa RM et al (2023) Astrocytic aerobic glycolysis provides lactate to support neuronal oxidative metabolism in the hippocampus. BioFactors. https://doi.org/10.1002/biof.1951
Pierre K, Pellerin L, Debernardi R et al (2000) Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 100:617–627. https://doi.org/10.1016/s0306-4522(00)00294-3
Rafiki A, Boulland JL, Halestrap AP et al (2003) Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience 122:677–688. https://doi.org/10.1016/S0306-4522(03)00654-7
Pierre K, Pellerin L (2005) Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem 94:1–14. https://doi.org/10.1111/j.1471-4159.2005.03168.x
Magistretti PJ, Allaman I (2018) Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci 19:235–249. https://doi.org/10.1038/nrn.2018.19
Bittar PG, Charnay Y, Pellerin L et al (1996) Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J Cereb Blood Flow Metab 16:1079–1089. https://doi.org/10.1097/00004647-199611000-00001
Laughton JD, Bittar P, Charnay Y et al (2007) Metabolic compartmentalization in the human cortex and hippocampus: evidence for a cell- and region-specific localization of lactate dehydrogenase 5 and pyruvate dehydrogenase. BMC Neurosci 8:35. https://doi.org/10.1186/1471-2202-8-35
Debernardi R, Pierre K, Lengacher S et al (2003) Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res 73:141–155. https://doi.org/10.1002/jnr.10660
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91:10625–10629. https://doi.org/10.1073/pnas.91.22.10625
Rosafio K, Castillo X, Hirt L, Pellerin L (2016) Cell-specific modulation of monocarboxylate transporter expression contributes to the metabolic reprograming taking place following cerebral ischemia. Neuroscience 317:108–120. https://doi.org/10.1016/j.neuroscience.2015.12.052
Zhang Y, Jia P, Wang K et al (2023) Lactate modulates microglial inflammatory responses after oxygen-glucose deprivation through HIF-1α-mediated inhibition of NF-κB. Brain Res Bull 195:1–13. https://doi.org/10.1016/j.brainresbull.2023.02.002
Salter MW, Stevens B (2017) Microglia emerge as central players in brain disease. Nat Med 23:1018–1027. https://doi.org/10.1038/nm.4397
Liddelow SA, Marsh SE, Stevens B (2020) Microglia and astrocytes in disease: dynamic duo or partners in crime? Trends Immunol 41:820–835. https://doi.org/10.1016/j.it.2020.07.006
Colucci ACM, Tassinari ID, da Silveira Loss E, de Fraga LS (2023) History and function of the lactate receptor GPR81/HCAR1 in the brain: a putative therapeutic target for the treatment of cerebral ischemia. Neuroscience 526:144–163. https://doi.org/10.1016/j.neuroscience.2023.06.022
Tassinari ID, de Fraga LS (2022) Potential use of lactate for the treatment of neonatal hypoxic-ischemic encephalopathy. Neural Regen Res 17:788–790. https://doi.org/10.4103/1673-5374.322459
Hoque R, Farooq A, Ghani A et al (2014) Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146:1763–1774. https://doi.org/10.1053/j.gastro.2014.03.014
Yang K, Xu J, Fan M et al (2020) Lactate suppresses macrophage pro-inflammatory response to LPS stimulation by inhibition of YAP and NF-κB activation via GPR81-mediated signaling. Front Immunol 11:587913. https://doi.org/10.3389/fimmu.2020.587913
Laroche S, Stil A, Germain P et al (2021) Participation of l-lactate and its receptor HCAR1/GPR81 in neurovisual development. Cells. https://doi.org/10.3390/cells10071640
Vohra R, Sanz-Morello B, Tams ALM et al (2022) Prevention of cell death by activation of hydroxycarboxylic acid receptor 1 (GPR81) in retinal explants. Cells. https://doi.org/10.3390/cells11132098
Griego E, Galván EJ (2023) BDNF and lactate as modulators of hippocampal CA3 network physiology. Cell Mol Neurobiol 43:4007–4022. https://doi.org/10.1007/s10571-023-01425-6
Vohra R, Aldana BI, Waagepetersen H et al (2019) Dual properties of lactate in Müller cells: the effect of GPR81 activation. Investig Ophthalmol Vis Sci 60:999–1008. https://doi.org/10.1167/iovs.18-25458
Herrera-López G, Griego E, Galván EJ (2020) Lactate induces synapse-specific potentiation on CA3 pyramidal cells of rat hippocampus. PLoS ONE 15:e0242309. https://doi.org/10.1371/journal.pone.0242309
Scavuzzo CJ, Rakotovao I, Dickson CT (2020) Differential effects of l-and d-lactate on memory encoding and consolidation: potential role of HCAR1 signaling. Neurobiol Learn Mem 168:107151. https://doi.org/10.1016/j.nlm.2019.107151
Buscemi L, Price M, Castillo-González J et al (2022) Lactate neuroprotection against transient ischemic brain injury in mice appears independent of HCAR1 activation. Metabolites. https://doi.org/10.3390/metabo12050465
Nicola R, Madar R, Okun E (2022) HCAR1-mediated l-lactate signaling suppresses microglial phagocytosis. Neuromol Med 24:399–404. https://doi.org/10.1007/s12017-022-08710-5
Kennedy L, Glesaaen ER, Palibrk V et al (2022) Lactate receptor HCAR1 regulates neurogenesis and microglia activation after neonatal hypoxia-ischemia. eLife. https://doi.org/10.7554/eLife.76451
Harun-Or-Rashid M, Inman DM (2018) Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. J Neuroinflamm 15:313. https://doi.org/10.1186/s12974-018-1346-7
Ahmed ME, Selvakumar GP, Kempuraj D et al (2019) Synergy in disruption of mitochondrial dynamics by Aβ (1-42) and glia maturation factor (GMF) in SH-SY5Y cells is mediated through alterations in fission and fusion proteins. Mol Neurobiol 56:6964–6975. https://doi.org/10.1007/s12035-019-1544-z
de-Brito NM, Duncan-Moretti J, da-Costa HC et al (2020) Aerobic glycolysis is a metabolic requirement to maintain the M2-like polarization of tumor-associated macrophages. Biochim Biophys Acta 1867:118604. https://doi.org/10.1016/j.bbamcr.2019.118604
Lee S-P, Chao S-C, Huang S-F et al (2018) Expressional and functional characterization of intracellular pH regulators and effects of ethanol in human oral epidermoid carcinoma cells. Cell Physiol Biochem 47:2056–2068. https://doi.org/10.1159/000491473
Halcrow P, Khan N, Datta G et al (2019) Importance of measuring endolysosome, cytosolic, and extracellular pH in understanding the pathogenesis of and possible treatments for glioblastoma multiforme. Cancer Rep. https://doi.org/10.1002/cnr2.1193
Paik S, Somvanshi RK, Kumar U (2019) Somatostatin-mediated changes in microtubule-associated proteins and retinoic acid-induced neurite outgrowth in SH-SY5Y cells. J Mol Neurosci 68:120–134. https://doi.org/10.1007/s12031-019-01291-2
Wölfle U, Haarhaus B, Kersten A et al (2015) Salicin from willow bark can modulate neurite outgrowth in human neuroblastoma SH-SY5Y cells. Phytother Res 29:1494–1500. https://doi.org/10.1002/ptr.5400
Cruz E, Bessières B, Magistretti P, Alberini CM (2022) Differential role of neuronal glucose and PFKFB3 in memory formation during development. Glia 70:2207–2231. https://doi.org/10.1002/glia.24248
Schurr A, Payne RS, Miller JJ, Rigor BM (1997) Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study. Brain Res 744:105–111. https://doi.org/10.1016/s0006-8993(96)01106-7
Wohnsland S, Bürgers HF, Kuschinsky W, Maurer MH (2010) Neurons and neuronal stem cells survive in glucose-free lactate and in high glucose cell culture medium during normoxia and anoxia. Neurochem Res 35:1635–1642. https://doi.org/10.1007/s11064-010-0224-1
Monsorno K, Ginggen K, Ivanov A et al (2023) Loss of microglial MCT4 leads to defective synaptic pruning and anxiety-like behavior in mice. Nat Commun 14:1–18. https://doi.org/10.1038/s41467-023-41502-4
McKenna MC, Hopkins IB, Carey A (2001) Alpha-cyano-4-hydroxycinnamate decreases both glucose and lactate metabolism in neurons and astrocytes: implications for lactate as an energy substrate for neurons. J Neurosci Res 66:747–754. https://doi.org/10.1002/jnr.10084
Dovmark TH, Saccomano M, Hulikova A et al (2017) Connexin-43 channels are a pathway for discharging lactate from glycolytic pancreatic ductal adenocarcinoma cells. Oncogene 36:4538–4550. https://doi.org/10.1038/onc.2017.71
Sotelo-Hitschfeld T, Niemeyer MI, Mächler P et al (2015) Channel-mediated lactate release by K+-stimulated astrocytes. J Neurosci 35:4168–4178. https://doi.org/10.1523/JNEUROSCI.5036-14.2015
Descalzi G, Gao V, Steinman MQ et al (2019) Lactate from astrocytes fuels learning-induced mRNA translation in excitatory and inhibitory neurons. Commun Biol 2:247. https://doi.org/10.1038/s42003-019-0495-2
Yang J, Yuan S, Jian Y et al (2023) Aerobic exercise regulates GPR81 signal pathway and mediates complement-microglia axis homeostasis on synaptic protection in the early stage of Alzheimer’s disease. Life Sci. https://doi.org/10.1016/j.lfs.2023.122042
Brooks GA (2020) Lactate as a fulcrum of metabolism. Redox Biol 35:101454. https://doi.org/10.1016/j.redox.2020.101454
Robergs RA, McNulty CR, Minett GM et al (2018) Lactate, not lactic acid, is produced by cellular cytosolic energy catabolism. Physiology 33:10–12
Blixt J, Song Y, Wanecek M, Gunnarson E (2023) EPO has multiple positive effects on astrocytes in an experimental model of ischemia. Brain Res 1802:148207. https://doi.org/10.1016/j.brainres.2022.148207
Beppu K, Sasaki T, Tanaka KF et al (2014) Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron 81:314–320. https://doi.org/10.1016/j.neuron.2013.11.011
Gupta P, Hourigan K, Jadhav S et al (2017) Effect of lactate and pH on mouse pluripotent stem cells: importance of media analysis. Biochem Eng J 118:25–33. https://doi.org/10.1016/j.bej.2016.11.005
Li H, Wang Y, Wang B et al (2021) Baicalin and geniposide inhibit polarization and inflammatory injury of OGD/R-treated microglia by suppressing the 5-LOX/LTB4 pathway. Neurochem Res 46:1844–1858. https://doi.org/10.1007/s11064-021-03305-1
Qin X, Sun Z-Q, Zhang X-W et al (2013) TLR4 signaling is involved in the protective effect of propofol in BV2 microglia against OGD/reoxygenation. J Physiol Biochem 69:707–718. https://doi.org/10.1007/s13105-013-0247-6
Zhang B-J, Men X-J, Lu Z-Q et al (2013) Splenectomy protects experimental rats from cerebral damage after stroke due to anti-inflammatory effects. Chin Med J 126:2354–2360. https://doi.org/10.3760/cma.j.issn.0366-6999.20122483
Unsicker C, Cristian F-B, von Hahn M et al (2021) SHANK2 mutations impair apoptosis, proliferation and neurite outgrowth during early neuronal differentiation in SH-SY5Y cells. Sci Rep 11:2128. https://doi.org/10.1038/s41598-021-81241-4
Martínez M-A, Rodríguez J-L, Lopez-Torres B et al (2020) Use of human neuroblastoma SH-SY5Y cells to evaluate glyphosate-induced effects on oxidative stress, neuronal development and cell death signaling pathways. Environ Int 135:105414. https://doi.org/10.1016/j.envint.2019.105414
Kovalevich J, Langford D (2013) Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol 1078:9–21. https://doi.org/10.1007/978-1-62703-640-5_2
Dravid A, Raos B, Svirskis D, O’Carroll SJ (2021) Optimised techniques for high-throughput screening of differentiated SH-SY5Y cells and application for neurite outgrowth assays. Sci Rep 11:23935. https://doi.org/10.1038/s41598-021-03442-1
Raghunath M, Patti R, Bannerman P et al (2000) A novel kinase, AATYK induces and promotes neuronal differentiation in a human neuroblastoma (SH-SY5Y) cell line. Brain Res Mol Brain Res 77:151–162. https://doi.org/10.1016/s0169-328x(00)00048-6
Al-Chalabi A, Miller CCJ (2003) Neurofilaments and neurological disease. BioEssays 25:346–355. https://doi.org/10.1002/bies.10251
Gao F, Wu J, Zhou Y et al (2020) An appropriate ratio of unsaturated fatty acids is the constituent of hickory nut extract for neurite outgrowth in human SH-SY5Y cells. Food Sci Nutr 8:6346–6356. https://doi.org/10.1002/fsn3.1623
Agrawal PB, Joshi M, Marinakis NS et al (2014) Expanding the phenotype associated with the NEFL mutation: neuromuscular disease in a family with overlapping myopathic and neurogenic findings. JAMA Neurol 71:1413–1420. https://doi.org/10.1001/jamaneurol.2014.1432
Hong H, Su J, Zhang Y et al (2023) A novel role of lactate: promotion of Akt-dependent elongation of microglial process. Int Immunopharmacol 119:110136. https://doi.org/10.1016/j.intimp.2023.110136
Li R, Yang Y, Wang H et al (2023) Lactate and lactylation in the brain: current progress and perspectives. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-023-01335-7
Zhang J, Muri J, Fitzgerald G et al (2020) Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab 31:1136–1153.e7. https://doi.org/10.1016/j.cmet.2020.05.004
Chaudhari P, Madaan A, Rivera JC et al (2022) Neuronal GPR81 regulates developmental brain angiogenesis and promotes brain recovery after a hypoxic ischemic insult. J Cereb Blood Flow Metab 42:1294–1308. https://doi.org/10.1177/0271678X221077499
Lauritzen KH, Morland C, Puchades M et al (2014) Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24:2784–2795. https://doi.org/10.1093/cercor/bht136
Morland C, Lauritzen KH, Puchades M et al (2015) The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. J Neurosci Res 93:1045–1055. https://doi.org/10.1002/jnr.23593
Acknowledgements
We want to express our gratitude to Dr. Amanda Thomaz from the Division of Biomedical and Life Sciences, Lancaster University, and also to the PhD student, Janaína Zang from the Federal University of Rio Grande do Sul (UFRGS) for their unwavering support in the immunofluorescence experiments.
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), National Institute of Science and Technology on Neuroimmunomodulation (INCT-NIM), FIOCRUZ Institutional Internationalization Program (CAPES/PrInt-Fiocruz), UFRGS Institutional Internationalization Program (CAPES/PrInt-UFRGS), and Erasmus+ Funding Programme.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: IDT, DAMC, RPG, AHP, VBJ, and LSF. Performed the experiments: IDT, and FSR. Analyzed the data: IDT, FSR, VBJ, and LSF. Data interpretation: IDT, DAMC, RPG, AHP, and LSF. Wrote the first draft of the manuscript: IDT, and LSF. Revised critically the manuscript: CB, DAMC, RPG, and AHP. Grammar review: CB. All authors reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
Institutional ethical approval was not required, as the present study used only cell lines, and live animals were not used in the experiments.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tassinari, I.D., Rodrigues, F.d., Bertram, C. et al. Lactate Protects Microglia and Neurons from Oxygen–Glucose Deprivation/Reoxygenation. Neurochem Res (2024). https://doi.org/10.1007/s11064-024-04135-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11064-024-04135-7