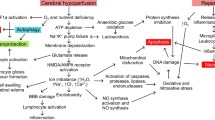
Abstract
The ability of insulin and gangliosides, administered separately or jointly, to increase the viability of cultured brain cortical neurons under conditions of oxidative stress and to normalize metabolic disorders in the rat brain cortex caused by the activation of free radical reactions after forebrain ischemia and subsequent reperfusion were studied. Preincubation of brain cortical neurons with insulin and/or brain ganglioside GM1 significantly increased the viability of the cells when exposed to hydrogen peroxide. Two-vessel forebrain ischemia–reperfusion caused an oxidative inactivation of Na+,K+-ATPase and significant elevation in various lipid peroxidation products. Both separate and joint administration of insulin (0.25 IU/rat, i.n.) or brain gangliosides (15 mg/kg, i.v.) prevented the accumulation of Schiff bases and significantly increased Na+,K+-ATPase activity in the ischemized and reperfused rat brain cortex. Co-administration of insulin and gangliosides at the above, relatively low, doses led to no reciprocal enhancement of the effects of both neuroprotectors. At the same time, the effect of intranasal insulin administration at a dose of 0.5 IU/rat was more pronounced than at 0.25 IU/rat. Insulin administration at a dose of 0.5 IU/rat normalized the levels of conjugated di- and trienes and Schiff bases, as well as Na+,K+-ATPase activity, in the ischemized and reperfused rat brain cortex. These findings indicate the ability of insulin and brain gangliosides to prevent or diminish a reduction in the number of survived cultured neurons under conditions of oxidative stress and metabolic disorders in the brain cortex caused by the activation of free radical reactions in the ischemized and reperfused rat forebrain, with no additivity observed in insulin and ganglioside effects.



Similar content being viewed by others
REFERENCES
Bachis A, Rabin SJ, Del Fiacco M, Mocchetti I (2002) Gangliosides prevent excitotoxicity through activation of TrkB receptor. Neurotox Res 4: 225–234. https://doi.org/10.1080/10298420290015836
Huang F, Dong X, Zhang L, Zhang X, Zhao D, Bai X, Li Z (2010) GM1 and nerve growth factor modulate mitochondrial membrane potential and neurofilament light mRNA expression in cultured dorsal root ganglion and spinal cord neurons during excitotoxic glutamate exposure. J Clin Neurosci 17: 495–500. https://doi.org/10.1016/j.jocn.2009.07.112
Avrova NF, Victorov IV, Tyurin VA, Zakharova IO, Sokolova TV, Andreeva NA, Stelmaschuk EV, Tyurina YY, Gonchar VS (1998) Inhibition of glutamate-induced intensification of free radical reactions by gangliosides: possible role in their protective effect in rat cerebellar granule cells and brain synaptosomes. Neurochem Res 23: 945–952. https://doi.org/10.1023/a:1021076220411
Zakharova IO, Sokolova TV, Vlasova YA, Furaev VV, Rychkova MP, Avrova NF (2014) GM1 ganglioside activates ERK1/2 and Akt downstream of Trk tyrosine kinase and protects PC12 cells against hydrogen peroxide toxicity. Neurochem Res 39: 2262–2275. https://doi.org/10.1007/s11064-014-1428-6
Ramalingam M, Kim SJ (2015) Insulin exerts neuroprotective effects via Akt/Bcl-2 signaling pathways in diffrentiated SH-SY5Y cells. J Recep Signal Transduct Res 35: 1–7. https://doi.org/10.3109/10799893.2014.922576
Zakharova IO, Sokolova TV, Bayunova LV, Zorina II. Rychkova MP, Shpakov AO, Avrova NF (2019) The protective effect of insulin on rat cortical neurons in oxidative stress and its dependence on the modulation of Akt, GSK-3beta, ERK1/2, and AMPK activities. Int J Mol Sci 20: E3702. https://doi.org/10.3109/10799893.2014.92257610.3390/ijms20153702
Chen Y, Guo Z, Mao YF, Zheng T, Zhang B (2018) Intranasal insulin ameliorates cerebral hypometabolism, neuronal loss, and astrogliosis in streptosotocin-induced Alzheimer’s rat model. Neurotox Res 33: 716–724. https://doi.org/10.1007/s12640-017-9809-7
Song Y, Ding W, Bei Y, Xiao Y, Tong HD, Wang LB, Ai LY (2018) Insulin is a potential antioxidant for diabetes-associated cognitive decline via regulating Nrf2 dependent antioxidant enzymes. Biomed Pharmacother 104: 474–484. https://doi.org/10.1016/j.biopha.2018.04.097
Fine JM, Stroebel BM, Faltesek KA, Terai K, Haase L, Knutzen KE, Kosyakovsky J, Bowe TJ, Fuller AK, Frey WH, Hanson LR (2020) Intranasal delivery of low-dose insulin ameliorates motor dysfunction and dopaminergic cell death in a 6-OHDA rat model of Parkinson’s Disease. Neurosci Lett 714: 134567. https://doi.org/10.1016/j.neulet.2019.134567
Claxton A, Baker LD, Hanson AJ, Trittschuh EH, Collerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S (2015) Long-acting insulin Detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis 44: 897–906. https://doi.org/10.3233/JAD-141791
Craft S, Claxton A, Baker LD, Hanson AJ, Collerton B, Trittschuh EH, Dahl D, Caulder E, Neth B, Montine TJ, Jung Y, Maldjian J, Whitlow C, Friedman S (2017) Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: A pilot clinical trial. J Alzheimers Dis 57: 1325–1334. https://doi.org/10.3233/JAD-161256
Avgerinos KI, Kalaitzidis G, Malli A, Kalaitzoglou D, Myserlis PG, Lioutas VA (2018) Intranasal insulin in Alzheimer’s dementia or mild cognitive impairment. A systematic review. J Neurol 265: 1497–1510. https://doi.org/10.1007/s00415-018-8768-0
Novak P, Maldonado DAP, Novak V (2019) Safety and preliminary efficacy of intranasal insulin for cognitive impairment in Parkinson disease and multiple system atrophy: A double-blinded placebo-controlled pilot study. PLoS One 14: e0214364. https://doi.org/10.1371/journal.pone.0214364
Hallschmid M (2021) Intranasal insulin. J Neuroendocrinol 33: e12934. https://doi.org/10.1111/jne.12934
Shpakov AO, Derkach KV, Berstein LM (2015) Brain signaling systems in the Type 2 diabetes and metabolic syndrome: promising target to treat and prevent these diseases. Future Sci OA 1: FSO25. https://doi.org/10.4155/fso.15.23
Romanova IV, Derkach KV, Mikhrina AL, Sukhov IB, Mikhailova EV, Shpakov AO (2018) The Leptin, Dopamine and Serotonin Receptors in Hypothalamic POMC-Neurons of Normal and Obese Rodents. Neurochem Res 43: 821–837. https://doi.org/10.1007/s11064-018-2485-z
Derkach K, Zakharova I, Zorina I, Bakhtyukov A, Romanova I, Bayunova L, Shpakov A (2019) The evidence of metabolic-improving effect of metformin in Ay/a mice with genetically-induced melanocortin obesity and the contribution of hypothalamic mechanisms to this effect. PLoS One. 14: e0213779. https://doi.org/10.1371/journal.pone.0213779
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB (2004) AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574. https://doi.org/10.1038/nature02440
Erichsen JM, Calva CB, Reagan, LP, Fadel JR (2021) Intranasal insulin and orexins to treat age-related cognitive decline. Physiol Behav 234: 113370. https://doi.org/10.1016/j.physbeh.2021.113370
Lochhead JJ, Kellohen KL, Ronaldson PT, Davis TP (2019) Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci Rep 9: 2621. https://doi.org/10.1038/s41598-019-39191-5
Fan LW, Carter K, Beatt A, Pang Y (2019) Rapid transport of insulin to the brain following intranasal administration in rats. Neural Regen Res 14: 1046–1051. https://doi.org/10.4103/1673-5374.250624
Tashima T (2020) Shortcut approaches to substance delivery into the brain based on intranasal administration using nanodelivery strategies for insulin. Molecules 25(21): 5188. https://doi.org/10.3390/molecules25215188
Zorina II, Galkina OV, Bayunova LV, Zakharova IO (2019) Effect of insulin on lipid peroxidation and glutathione levels in a two-vessel occlusion model of rat forebrain ischemia followed by reperfusion. J Evol Biochem Physiol 35: 333–335. https://doi.org/10.1134/S0022093019040094
Zorina II, Fokina EA, Zakharova IO, Bayunova LV, Shpakov AO (2020) Characteristics of changes in lipid peroxidation and Na+/K+-ATPase activity in the cortex of old rats in conditions of two-vessel cerebral ischemia/reperfusion. Adv Geront 10: 156–161. https://doi.org/10.1134/s2079057020020162
Kooijman R, Sarre S, Michotte Y, De Keyser J (2009) Insulin-like growth factor-I: a potential neuroprotective compound for the treatment of acute ischemic stroke? Stroke 40: e83–e88. https://doi.org/10.1161/STROKEAHA.108.528356
Shen H, Gu X, Wei ZZ, Wu A, Liu X (2021) Combinatorial intranasal delivery of bone marrow mesenchymal stem cells and insulin-like growth factor-1 improves neurovascularization and functional outcomes following focal cerebral ischemia in mice. Exp Neurol 337: 113542. https://doi.org/10.1016/j.expneurol.2020.113542
Lioutas VA, Alfaro-Martinez F, Bedoya F, Chung CC, Pimentel DA, Novak V (2015) Intranasal insulin and insulin-like growth factor-1 as neuroprotectants in acute ischemic stroke. Transl Stroke Res 6: 264–275. https://doi.org/10.1007/s12975-015-0409-7
Sanderson TH, Kumar R, Murariu-Dobrin AC, Page AB, Krause GS, Sullivan JM (2009) Insulin activates the PI3K-Akt survival pathway in vulnerable neurons following global brain ischemia. Neurol Res 31: 947–958. https://doi.org/10.1179/174313209X382449
Huang SS, Lu YJ, Huang JP., Wu YT, Day YJ, Hung LM (2014) The essential role of endothelial nitric oxide synthase activation in insulin-mediated neuroprotection against ischemic stroke in diabetes. J Vasc Surg 59: 483–491. https://doi.org/10.1016/j.jvs.2013.03.023
Russo V, Candeloro P, Malara N, Perozziello G, Iannone M, Scicchitano M, Mollace R, Musolino V, Gliozzi M, Carresi C, Morittu VM, Gratteri S, Palma E, Muscoli C, Di Fabrizio E, Mollace V (2019) Key role of cytochrome C for apoptosis detection using Raman microimaging in an animal model of brain ischemia with insulin treatment. Appl Spectrosc 73: 1208–1217. https://doi.org/10.1177/0003702819858671
Su D, Ma J, Yang J, Kang Y, Lv M, Li Y (2017) Monosialotetrahexosyl-1 ganglioside attenuates diabetes-associated cerebral ischemia/reperfusion injury through suppression of the endoplasmic reticulum stress-induced apoptosis. J Clin Neurosci 41: 54–59. https://doi.org/10.1016/j.jocn.2017.03.047
Schneider JS, Aras R, Williams CK, Koprich JB, Brotchie JM, Singh V (2019) GM1 ganglioside model of Parkinson’s disease. Sci Rep 9: 8362. https://doi.org/10.1038/s41598-019-42847-x
Zhang Z, Liu W, Shen M, Ma X, Li R, Jin X, Bai H, Gao L (2021) Protective effect of GM1 attenuates hippocampus and cortex apoptosis after ketamine exposure in neonatal rat via PI3K/AKT/GSK3β pathway. Mol Neurobiol 58: 3471–3483. https://doi.org/10.1007/s12035-021-02346-5
Yamamoto HA, Mohanan PV (2003) Ganglioside GT1b and melatonin inhibit brain mitochondrial DNA damage and seizures induced by kainic acid in mice. Brain Res 964: 100–106. https://doi.org/10.1016/s0006-8993(02)04083-0
Zhang J, Fang X, Zhou Y, Deng X, Lu Y, Li J, Li S, Wang B, Xu R (2015) The possible damaged mechanism and the preventive effect of monosialotetrahexosylganglioside in a rat model of cerebral ischemia-reperfusion injury. J Stroke Cerebrovasc Dis 24: 1471–14788. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.02.008
Mao L, Liao YJ, Hou GH, Yang ZB, Zuo ML (2016) Monosialotetrahexosylganglioside protect cerebral ischemia/reperfusion injury through upregulating the expression of tyrosine hydroxylase by inhibiting lipid peroxidation. Biomed Pharmacother 84: 1923–1929. https://doi.org/10.1016/j.biopha.2016.11.019
Mironova EV, Evstratova AA, Antonov SM (2007) A fluorescence vital assay for the recognition and quantification of excitotoxic cell death by necrosis and apoptosis using confocal microscopy on neurons in culture J Neurosci Methods 163: 1–8. https://doi.org/10.1016/j.jneumeth.2007.02.010
Zorina II, Bayunova LV, Zakharova IO, Avrova NF (2018)The dependence of the protective effect of insulin on its concentration and modulation of ERK1/2 activity under the conditions of oxidative stress in cortical neurons. Neurochem J 12: 111–116. https://doi.org/10.1134/S1819712417040110
Molchanova SM, Moskvin AN, Zakharova IO, Yurlova LA, Nosova IY, Avrova NF (2005) The effect of two-vessel forebrain ischemia and administration of indomethacin and quinacrine on Na+, K+-ATPase activity in different areas of the rat brain. J Evol Biochem Physiol 41: 33–38.
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509.
Ferenczi S, Kuti D, Cserháti M, Krifaton C, Szoboszlay S, Kukolya J, Szőke Z, Albert M, Kriszt B, Kovács KJ, Mézes M, Balogh K (2020) Effects of single and repeated oral doses of ochratoxin A on the lipid peroxidation and antioxidant defense systems in mouse kidneys. Toxins (Basel) 12: 732. https://doi.org/10.3390/toxins12110732
Sarieva, KV, Lyanguzov AY, Galkina OV, Vetrovoy OV (2019) The effect of severe hypoxia on HIF1- and Nrf2-mediated mechanisms of antioxidant defense in the rat neocortex. Neurochem J 13: 145–155. https://doi.org/10.1134/S1819712419020107
Leon A, Facci L, Toffano G, Sonnino S, Tettamanti G (1981) Activation of Na+, K+-ATPase by nanomolar concentrations of GM1 ganglioside. J Neurochem 37: 350–357. https://doi.org/10.1111/j.1471-4159.1981.tb00462.x
Pasantes-Morales H, Tuz K (2006) Volume changes in neurons: hyperexcitability and neuronal death. Contrib Nephrol 152: 221–240. https://doi.org/10.1159/000096326
Shao LR, Janicot R, Stafstrom CE (2021) Na+-K+-ATPase functions in the developing hippocampus: regional differences in CA1 and CA3 neuronal excitability and role in epileptiform network bursting. J Neurophysiol 125: 1–11. https://doi.org/10.1152/jn.00453.2020
Kadoya A, Miyake H, Ohyashiki T (2003) Contribution of lipid dynamics on the inhibition of bovine brain synaptosomal Na+-K+-ATPase activity induced by 4-hydroxy-2-nonenal. Biol Pharm Bull 26: 787–793. https://doi.org/10.1248/bpb.26.787
Miyake H, Kadoya A, Ohyashiki T (2003) Increase in molecular rigidity of the protein conformation of brain Na+-K+-ATPase by modification with 4-hydroxy-2-nonenal. Biol Pharm Bull 26: 1652–1656. https://doi.org/10.1248/bpb.26.1652
Abdalla FH, Schmatz R, Cardoso AM, Carvalho FB, Baldissarelli J, de Oliveira JS, Rosa MM, Goncalves Nunes MA, Rubin MA, da Cruz IB, Barbisan F, Dressler VL, Pereira LB, Schetinger MR, Morsch VM, Gonçalves JF, Mazzanti CM (2014) Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na+,K+-ATPase activities. Physiol Behav 135: 152–167. https://doi.org/10.1016/j.physbeh.2014.06.008
Hendrickx JO, Moudt S, Calus E, Martinet W, Pieter-Jan DF, Guns P-JD, Roth L, Peter P, De Deyn PP, Debby Van Dam D, De Meyer GRY (2021) Serum corticosterone and insulin resistance as early biomarkers in the hAPP23 overexpressing mouse model of Alzheimer’s disease. Int J Mol Sci 22: 6656. https://doi.org/10.3390/ijms22136656
Funding
This work was supported by the assignment of the Ministry of Science and Higher Education of the Russian Federation to the Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences (AAAA-A18-118012290427-7).
Author information
Authors and Affiliations
Contributions
Conceptualization (A.O.S., N.F.A., I.O.Z.); methodology (N.F.A., A.O.S., L.V.B.); validation (L.V.B.); investigation (I.O.Z., L.V.B., I.I.Z.); writing and editing (N.F.A., I.O.Z., A.O.S., L.V.B.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have neither evident nor potential conflict of interest in relation with the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 2, pp. 262–278https://doi.org/10.31857/S086981392202011X.
Rights and permissions
About this article
Cite this article
Zakharova, I.O., Bayunova, L.V., Zorina, I.I. et al. Insulin and Brain Gangliosides Prevent Metabolic Disorders Caused by Activation of Free Radical Reactions after Two-Vessel Ischemia–Reperfusion Injury to the Rat Forebrain. J Evol Biochem Phys 58, 279–291 (2022). https://doi.org/10.1134/S0022093022010240
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022010240