Abstract
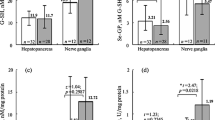
In experiments on mollusc Lymnaea stagnalis, the state of antioxidative protection is studied in central nervous ganglia during a long-term activation (inhibition) of synthesis of nitrogen monoxide (NO) in the body. The effect of the blocker of NO-synthase NG-nitro-L-arginine (L-NNA) at the background of enhancement of pulmonary respiration has been found to be associated with a rise of levels of reduced glutathione and TBK-active products in the nervous tissue at preservation of a relatively high superoxide dismutase activity and a low glutathione peroxidase activity compared with the control group and the animals treated with the metabolic precursor of NO synthesis L-arginine. In spite of the revealed disturbances of balance of the body proand antioxidative system, DNA electrophoresis detected no products of its degradation, which can indicate the absence of massive programmed death of the nervous tissue cells in Lymnaea stagnalis during modulation of activity of the NO-ergic system.
Similar content being viewed by others
References
Vincent, S.R., Nitric Oxide: a Radical Neurotransmitter in the Central Nervous System, Progr. Neurobiol., 1994, vol. 42, pp. 129–160.
Moroz, L.L. and Gilletee, R., From Polyplacophora to Cephalopoda: Comparative Analysis of Nitric Oxide Signalling in Mollusca, Acta Biol. Hung., 1995, vol. 46, pp. 169–182.
Bredt, D.S. and Snyder, S.H., Nitric Oxide, a Novel Neuronal Messenger, Neuron, 1992, vol. 8, pp. 3–11.
Blough, N.V. and Zafiriou, O.C., Reaction of Superoxide with Nitric-Oxide to Form Peroxonitrite in Alkaline Aqueous-Solution, Inorg. Chem., 1985, vol. 24, pp. 3502–3504.
Dubinina, E.E., Role of the Active Oxygen Forms as Signaling Molecules in Tissue Metabolism at States of Oxidative Stress, Vopr. Med. Khim., 2001, vol. 47, pp. 561–581.
Beckman, K. and Ames, B.N., The Free Radical Theory of Aging Matures, Physiol. Rev., 1998, vol. 78, pp. 547–581.
Moroz, L.L., Winlow, W., Turner, R.W., Bulloch, A.G., Lukowiak, K., and Syed, N.I., Nitric Oxide Synthase-Immunoreactive Cells in the CNS and Periphery of Lymnaea, NeuroReport, 1994, vol. 5, pp. 1277–1280.
Moroz, L.L., Park, J.-H., and Winlow, W., Nitric Oxide Activates Buccal Motor Patterns in Lymnaea stagnalis, NeuroReport, 1993, vol. 4, pp. 643–646.
Moroz, L.L. and Park, J.-H., Nitric Oxide Modulates the Central Respiratory Patterns in Lymnaea stagnalis, J. Physiol., 1993, vol. 473, p. 188.
Kazakevich, V.B., Sidorov, A.V., and Gurin, V.N., Nitroden Monoxide Coordinates the Digestive and Protective Behavior of Lymnaea stagnalis, Vestsi NAS of Belarus, Ser. Biol. Navuk, 2002, no. 1, pp. 73–75.
Porte, C., Sole, M., Albaiges, J., and Livingstone, D.R., Responses of Mixed-Function Oxygenase and Antioxidase Enzyme System of Mytilus sp. to Organic Pollution, Comp. Biochem. Physiol., 1991, vol. 100C, pp. 183–186.
Geret, F., Serafim, A., and Bebianno, M.J., Antioxidant Enzyme Activities, Metallothioneins and Lipid Peroxidation as Biomarkers in Ruditapes decussates, Ecotoxicol., 2003, vol. 12, pp. 417–426.
Kostyuk, V.A. and Potapovich, A.I., SuperoxideDriven Oxidation of Quercetin and a Simple Assay for Determination of Superoxide Dismutase, Biochem. Int., 1989, vol. 19, pp. 1117–1124.
Flohe, L. and Cunzler, W.A., Assays of Glutathione Peroxidase, Meth. Enzymol., 1984, vol. 105, pp. 114–126.
Habeeb, A.F., Reaction of Protein Sulfhydryl Groups with Ellman’s Reagent, Meth. Enzymol., 1972, vol. 25, pp. 457–464.
Kostyuk, V.A. and Potapovich, A.I., Determination of Products of Lipid Peroxidation Using Thiobarbituric Acid under Anaerobic Conditions, Vopr. Med. Khim., 1987, vol. 33, pp. 115–118.
Bradford, M.M., A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Hermes-Lima, M. and Storey, K.B., Antioxidant Defences and Metabolic Depression in a Pulmonate Land Snail, Am. J. Physiol., 1955, vol. 268, pp. 1386–1393.
Ramos-Vasconcelos, G.R. and Hermes-Lima, M., Hypometabolism, Antioxidant Defences and Free Radical Metabolism in the Pulmonate Land Snail Helix aspersa, J. Exp. Biol., 2003, vol. 206, pp. 675–684.
Santovito, G., Piccinni, E., Cassini, A., Irato, P., and Albergoni, V., Antioxidant Responses of the Mediterranean mussel, Mytilus galloprovincialis, to Environmental Variability of Dissolved Oxygen, Comp. Biochem. Physiol., 2005, vol. 140, pp. 321–329.
Kobayashi, S., Sadamoto, H., Ogawa, H., Kitamura, Y., Oka, K., Tanishita, K., and Ito, E., Nitric Oxide Generation around Buccal Ganglia Accompanying Feeding Behaviour in the Pond Snail, Lymnaea stagnalis, Neurosci. Res., 2000, vol. 38, pp. 27–34.
Faller, D.M. and Shields, D., Molekulyarnaya biologiys kletki (Molecular Biology of the Cell), Moscow, 2003.
Zielinski, S. and Portner, H.O., Oxidative Stress and Antioxidative Defense in Cephalopods: A Function of Metabolic Rate or Age?, Comp. Biochem. Physiol., 2000, vol. 125B, pp. 147–160.
Han, Y.T., Han, Z.W., Yu, G.Y., Wang, Y.J., Cui, R.Y., and Wang, C.B., Inhibitory Effect of Polypeptide from Chlamys farreri on Ultraviolet AInduced Oxidative Damage on Human Skin Fibroblasts in vitro, Pharmacol. Res., 2004, vol. 49, pp. 256–274.
Leng, B., Liu, X.D., and Chen, Q.X., Inhibitory Effects of Anti-Cancer Peptide from Mercenaria on the BGC-823 Cells and Several Enzymes, FEBS Lett., 2005, vol. 579, pp. 1187–1190.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A. V. Sidorov, G. T. Maslova, 2008, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2008, Vol. 44, No. 5, pp. 453–458.
Rights and permissions
About this article
Cite this article
Sidorov, A.V., Maslova, G.T. State of antioxidative protection in central nervous ganglia of the mollusc Lymnaea stagnalis at modulation of activity of the NO-ergic system. J Evol Biochem Phys 44, 535–541 (2008). https://doi.org/10.1134/S0022093008050010
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093008050010