Using the spectroscopic data on the 235UF6 and 238UF6 molecules and on the lasing frequencies of CF4 and para-H2 lasers and recent results, a method has been proposed to increase the efficiency of the isotope-selective infrared laser dissociation of 235UF6 molecules under nonequilibrium thermodynamic shock conditions. The method involves two processes: (i) the resonant multiphoton excitation of 235UF6 molecules to the 3ν3 or 2ν3 vibrational states by the bichromatic infrared radiation of two CF4 or para-H2 lasers and (ii) the irradiation of 235UF6 molecules with SF6 molecules serving as sensitizers resonantly absorbing the radiation of these lasers. The essence of the method has been described. Schemes and parameters for isotope-selective dissociation of 235UF6 molecules using this method has been presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Numerous recent studies have been devoted to molecular laser isotope separation (MLIS) [1–23]. The main aim of many studies is to develop uranium isotope separation methods involving UF6 molecules [1–7, 10–22]. Most of the laser uranium isotope separation projects carried out in the United States, Germany, United Kingdom, France, Japan, and Australia at the end of the past century were closed. At the same time, studies on uranium MLIS are currently performed in many countries [1–7, 20–22]. It is expected that the application of lasers will allow the development of a laser uranium enrichment technology more economical and efficient than centrifuging. Current research is focused on the development of low-energy MLIS methods [1–3, 10–22] and alternative methods [2, 3, 13, 24, 25].
Two important problems of laser separation of uranium isotopes involving UF6 molecules are (i) a small isotopic shift in infrared absorption spectra of laser-excited ν3 vibrations of 235UF6 and 238UF6 molecules and (ii) the absence of intense and efficient laser sources for isotope-selective vibrational excitation and dissociation of UF6 molecules. The isotopic shift in the ν3 vibrational mode (\( \approx {\kern 1pt} 627.7\) cm–1 [26]) for 235UF6 and 238UF6 molecules is \(\Delta {{\nu }_{{{\text{is}}}}} \approx 0.604{\kern 1pt} \) cm–1 [26]. Because of a small isotopic shift and a comparatively large width (about 20 cm–1) of the infrared absorption band of molecules at room temperature [2, 27], isotope-selective infrared dissociation of UF6 molecules is possible only at a low temperature of the gas when the infrared absorption band of UF6 molecules with different U isotopes is much narrower [1, 2, 27].
Two tunable radiation sources in the 16 μm range were developed for projects on uranium MLIS using isotope-selective infrared multiphoton dissociation of UF6 molecules. These are a molecular CF4 laser optically pumped by the intense radiation of a CO2 laser [28, 29] and the so-called para-H2 laser source based on the shift of the radiation frequency of the CO2 laser to the 16 μm range caused by the stimulated Raman scattering on rotational transitions of para-hydrogen molecules [30, 31]. These lasers in many parameters satisfy the requirements for operation at megafacilities [2]. However, significant disadvantages of both CF4 and para-H2 lasers in application to uranium isotope separation are discreteness of the frequency tuning and the absence of strong tunable lasing lines in the region of the Q branch of the ν3 vibrational mode of 235UF6 molecules (in the 628.32 cm–1 range [26]).
As a possible approach to uranium MLIS, we consider isotope-selective excitation of 3ν3 states of 235UF6 molecules (≈1877.5 cm–1 [32]), where the isotopic shift is about 1.81 cm–1 [26], by the radiation of a CO laser [33–36]. This approach involves the chemical reaction of vibrationally excited UF6 molecules with HCl molecules [33, 34]. Uranium isotopes are separated because the reaction rates of vibrationally excited and unexcited UF6 molecules with HCl molecules are different. For this approach, high-power CO lasers [35, 36] generating in the 5.3 μm range are being produced; they are planned to be used to excite 235UF6 molecules. However, the efficient excitation of 3ν3 states of UF6 molecules by infrared radiation with a wavelength of ≈5.3 μm is problematic because abs-orption of UF6 molecules at the \(0{{\nu }_{3}} \to 3{{\nu }_{3}}\) vibrational transition is weak. The integral absorption of the overtone \(0{{\nu }_{3}} \to 3{{\nu }_{3}}\) band of UF6 molecules is about a factor of 1.8 × 104 lower than the integral absorption of the main \(0{{\nu }_{3}} \to 1{{\nu }_{3}}\) band of UF6 molecules [32]. Consequently, the search for alternative schemes for isotope-selective excitation and dissociation of 235UF6 molecules is a very important relevant task. In this work, we propose a new method for the efficient isotope-selective laser infrared dissociation of 235UF6 molecules.
2 FOUNDATIONS OF THE METHOD
The proposed method is based on the process of resonant three- or two-photon excitation of 235UF6 molecules to the 3ν3 or 2ν3 vibrational states by the bichromatic infrared radiation of two pulsed CF4 or para-H2 lasers [18, 19] and on the irradiation of excited 235UF6 molecules with SF6 molecules serving as sensitizers resonantly absorbing the radiation of these lasers [16, 17, 23]. In addition, it is proposed to perform the excitation of molecules under nonequilibrium thermodynamic shock conditions formed in front of a solid surface on which a gas-dynamically cooled intense supersonic molecular flow is incident [1, 2, 24, 25].
As shown in [37, 38], radiation pulses of the CO2 laser can efficiently excite SF6 molecules to the high 2ν3 and 3ν3 vibrational states by means of the resonant two- [37] and three-frequency [38] excitation of SF6 molecules cooled in a gas-dynamic jet. Recent studies [16, 23] demonstrate the possibility of a strong increase in the dissociation yield of CF2HCl molecules irradiated under shock conditions together with CF3Br sensitizer molecules resonantly absorbing the laser radiation. It was established that the processes mentioned above are also applicable to other molecules [16, 23, 37], in particular, UF6 molecules. These processes are involved in the proposed method implemented under nonequilibrium thermodynamic shock conditions.
To form the molecular flow, it is proposed to use the UF6/SF6/CH4 molecular mixture with a pressure ratio of about 1/3/10 [39], where SF6 molecules serve as sensitizers and CH4 molecules are used as acceptors of F atoms produced in the dissociation of UF6 and SF6 molecules. At the indicated pressure ratio of used gases, the vibrational temperature of UF6 molecules (and SF6 molecules) in the flow incident on the surface and in the compression shock will be \({{T}_{{{\text{vib}}}}} \leqslant 100{\kern 1pt} \)K [27, 39]. The population of the ground vibrational state of UF6 molecules at this vibrational temperature is about 50% [2, 27], and UF6 molecules have comparatively narrow (a FWHM of about 7–8 cm–1) infrared absorption bands [39].
The same laser pulses exciting 235UF6 molecules also resonantly excite SF6 molecules used as sensitizers. The frequency of the ν4 vibrational mode of SF6 molecules (≈ 615 cm–1 [40, 41]) is in very good resonance with high-lying transitions of vibrationally excited 235UF6 molecules. For this reason, the energy is efficiently transferred from SF6 molecules excited by the CF4 or para-H2 lasers to vibrationally excited 235UF6 molecules. As a result, the efficient isotope-selective dissociation of 235UF6 molecules is ensured by radiative and collisional excitation processes [16, 17, 23].
It is noteworthy that SF6 molecules were used as sensitizers for the excitation and dissociation of UF6 molecules in many works (see, e.g., [2, 42] and references therein). However, SF6 molecules were used in those works for the preliminary accumulation of the vibrational energy through their excitation by the pulsed radiation of the CO2 laser. SF6 molecules have an intense absorption band in the 10.6 μm range (ν3 vibrational mode at a frequency of ≈948 cm–1 [43]). Subsequently, the energy accumulated by SF6 molecules was transferred to the ν3 mode of UF6 molecules through the ν4 mode resonant to the f-ormer, which led to their excitation and dissociation [2, 42].
Unlike the cited works, sensitizer and 235UF6 molecules in the proposed method should be excited simultaneously by the resonant radiation of CF4 or para-H2 lasers, which significantly increases the efficiency of the dissociation of 235UF6 molecules [16, 17, 23]. Since the dissociation energy of UF6 molecules (≈68 kcal/mol [44]) is much lower than that of SF6 molecules (≈92 kcal/mol [45]), the dissociation of UF6 molecules in the process of irradiation of the mixture will occur at a much lower vibrational temperature than the dissociation of SF6 molecules. As a result, under certain conditions possible at a low fl-uence of excited laser radiation (\(\Phi \leqslant 1.5{-} 2\) J/cm2), UF6 molecules will be dissociated, whereas the dissociation of SF6 molecules will not occur [16, 17, 23].
The translational, rotational, and vibrational temperatures of polyatomic molecules in the gas-dynamically cooled molecular flow are related as [46] \({{T}_{{1,{\text{tr}}}}} \leqslant {{T}_{{1,{\text{rot}}}}} \leqslant {{T}_{{1,{\text{vib}}}}}\). In the compression shock [47], because of the different translational, rotational, and vibrational relaxation rates [48], inverse nonequilibrium relation \({{T}_{{2,{\text{tr}}}}} \geqslant {{T}_{{2,{\text{rot}}}}} \geqslant {{T}_{{2,{\text{vib}}}}}\) occurs [1, 2, 24]. The subscripts 1 and 2 indicate the temperatures of molecules in the incident flow and compression shock, respectively. In this case, because of a long vibrational–translational relaxation time of molecules (e.g., \(p{{\tau }_{{{\text{V}} - {\text{T}}}}} \approx 150\) μs Torr for SF6 [49], \(p{{\tau }_{{{\text{V}} - {\text{T}}}}} \approx \) 32 μs Torr for UF6 [50]), the vibrational temperature of molecules in the compression shock in the case of the pulsed rarefied gas flow can be approximately equal to the vibrational temperature of molecules in the incident flow \(({{T}_{{2,{\text{vib}}}}} \approx {{T}_{{1,{\text{vib}}}}})\), whereas the translational and rotational temperatures of molecules in the compression shock are much higher than the respective temperatures in the unperturbed flow: \({{T}_{{2,{\text{tr}}}}} > {{T}_{{1,{\text{tr}}}}}\) and \({{T}_{{2,{\text{rot}}}}} > {{T}_{{1,{\text{rot}}}}}\). Thus, new nonequilibrium conditions are formed in the compression shock, where the vibrational temperature of molecules is much lower than their translational and rotational temperatures.
For the resonant excitation of the 2ν3 and 3ν3 vibrational states of 235UF6 molecules by the radiation of two pulsed infrared lasers, it is necessary [51] to satisfy the following relations between the frequencies \({{\nu }_{{{\text{1L}}}}}\) and \({{\nu }_{{{\text{2L}}}}}\) of these lasers and the frequency ν3 of the excited vibrational mode of 235UF6 molecules:
One can quite easily ensure resonance conditions for the excitation of high vibrational levels of molecules using two or three lasers with different frequencies, particularly, high-pressure lasers with smooth radiation frequency tuning. To carry out such experiments, it is necessary to exactly know the frequencies (energies) of high vibrational levels of the studied molecules. These data for the SF6 [52] and UF6 [53] molecules were obtained at the Los Alamos National Laboratory in the course of projects on the molecular laser separation of uranium isotopes.
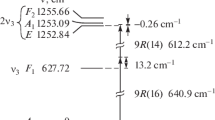
Advantages of the irradiation of 235UF6 molecules with an SF6 sensitizer resonantly absorbing laser radiation to increase the efficiency of their dissociation are illustrated in Fig. 1. This figure shows the infrared absorption band of the ν4 vibrational mode (frequency of 615 cm–1 [41, 54]) of molecules of SF6 resonantly absorbing the radiation of the CF4 laser at a temperature of \(T = 213{\kern 1pt} \) K [54] and the infrared absorption band of the ν3 vibrational mode (\( \approx \)627.72 cm–1 [26]) of UF6 molecules cooled in a supersonic gas-dynamic jet in a mixture with argon at a temperature of \(T \leqslant 130{\kern 1pt} \)K [39]. The vertical arrows in Fig. 1 mark the frequencies of 618.2 and 640.9 cm–1 of the lasing lines of the CF4 laser at which it is proposed to excite 235UF6 molecules to the 3ν3 F\(_{1}\) (1877.41 cm–1) state (see below). The horizontal arrows indicate the direction of the redshift of the absorption bands of the UF6 and SF6 molecules under their vibrational excitation.
(Color online) Infrared absorption band of the ν4 mode of SF6 molecules at a temperature of \(T = 213{\kern 1pt} \) K [54] and the infrared absorption band of the ν3 mode of UF6 molecules cooled in a supersonic gas-dynamic jet in a mixture with argon at a temperature of \(T \leqslant 130{\kern 1pt} \) K [39]. The vertical arrows mark the frequency positions of the lasing lines of the CF4 laser at which it is proposed to excite 235UF6 molecules to the 3ν3 F1 state (1877.41 cm–1). The horizontal arrows indicate the direction of the redshift of the absorption bands of the UF6 and SF6 molecules under their vibrational excitation.
Because of the anharmonicity of vibrations, the resonant excitation of 235UF6 molecules to the 2ν3 and 3ν3 vibrational states by bichromatic infrared laser radiation leads to the shift of their infrared absorption band to the low-frequency range coinciding with the infrared absorption band of the ν4 vibrational mode of SF6 molecules. As a result, the effective resonant radiation–collisional excitation of vibrationally excited 235UF6 molecules and SF6 molecules occurs [16, 17, 23, 42]. Laser-excited SF6 molecules transfer the absorbed energy to 235UF6 molecules through the vibrational–vibrational (V–V) energy exchange, increasing their dissociation yield. The process of V–V energy exchange between molecules is highly efficient because it occurs at small frequency detuning between vibrational transitions in SF6 and UF6 molecules [42, 55]. This high efficiency is also due to a comparatively high density of particles in the compression shock [≈(5–7) × 1016 [23, 24]] and high vibrational and rotational temperatures of molecules (≥550 K [23, 24]).
3 SCHEMES AND PARAMETERS FOR RESONANT EXCITATION OF THE 2ν3 AND 3ν3 STATES OF 235UF6 MOLECULES
We calculated the 2ν3 and 3ν3 states of 235UF6 molecules taking into account the isotopic shift \(\Delta {{\nu }_{{{\text{is}}}}} = 0.604{\kern 1pt} \)cm–1 [26] in the absorption band of the ν3 vibrational mode for 235UF6 and 238UF6. The isotopic shift in the 2ν3 and 3ν3 states is taken as 1.21 and 1.81 cm–1, respectively. The 2ν3 and 3ν3 energy levels of 235UF6 molecules are shifted by these values toward higher energies. Two infrared lasers allow the efficient isotope-selective excitation of both 2ν3 and 3ν3 vibrational states of 235UF6 and 238UF6 molecules [18, 19]. We consider the excitation of only those 2ν3 and 3ν3 states of 235UF6 molecules through which 235UF6 molecules can be resonantly excited to higher 4ν3 and 6ν3 vibrational states.
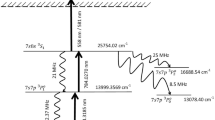
Figure 2а shows the scheme of excitation of the 3ν3 F1 vibrational state of 235UF6 molecules (1877.41 cm–1 [53]) by radiation of two CF4 lasers at frequencies of \({{\nu }_{{{\text{L1}}}}} \approx 618.2{\kern 1pt} \) cm–1 and \({{\nu }_{{{\text{L2}}}}} \approx 640.9{\kern 1pt} \) cm–1. The three-photon bichromatic excitation of the 3ν3 F\(_{1}\) level is performed with the detuning in the final state \(\Delta {{\nu }_{{{\text{fin}}}}} \approx 0.11{\kern 1pt} \) cm–1 (2νL1 + νL2 = 1877.3 cm–1). For this case, the solid arrows in Fig. 2b mark the frequencies of the lasing lines of the CF4 lasers with respect to Q branches of the ν3 mode of 238UF6 and 235UF6 molecules in the gas-dynamically cooled molecular flow at a temperature of \(T \leqslant 50{-} 70\) K [27]. The solid arrows in Fig. 2b mark the frequencies of the lasing lines of the para-H2 lasers for the excitation of the 3ν3 F1 state of 235UF6 molecules. When choosing the schemes for resonant excitation of molecules, we took into account only the most intense radiation lines of the CF4 [56] and para-H2 lasers. The lasing frequencies of the lasers are neither individually nor pairwise in resonance with low-lying transitions in UF6 molecules.
(а) Scheme of the resonant three-photon bichromatic excitation of the 3ν3 F1 state (1877.41 cm–1 [53]) of 235UF6 molecules by the radiation of two CF4 lasers. (b) (Solid arrows) Frequency positions of lasing lines of the CF4 lasers for the excitation of the 3ν3 F1 state of 235UF6 molecules with respect to the Q branches of the ν3 mode of 238UF6 and 235UF6 molecules in the gas-dynamically cooled molecular flow at a temperature of \(T \leqslant 50{-} 70\) K [27] and (dashed arrows) frequency positions of lasing lines of the para-H2 lasers for the excitation of the 3ν3 F1 state of 235UF6 molecules.
Possible schemes proposed for the resonant two-photon bichromatic excitation of 2ν3 vibrational states of 235UF6 molecules by the infrared radiation of two CF4 lasers and two para-H2 lasers are summarized in Table 1. Table 2 presents the schemes proposed for the resonant three-photon bichromatic excitation of 3ν3 vibrational states of 235UF6 molecules by the infrared radiation of two CF4 lasers and two para-H2 lasers. According to Tables 1 and 2, both types of lasers can provide the resonant excitation of the 2ν3 and 3ν3 states of 235UF6 molecules with a small frequency detuning in the final state, which promotes a high selectivity of the excitation of 235UF6 molecules. In all schemes presented in Tables 1 and 2 for the excitation of the 2ν3 and 3ν3 states of 235UF6 molecules, the 4ν3 and 6ν3 states of 235UF6 molecules can also be resonantly populated by the same laser pulses. This possibility is valuable because this ensures [57] a more efficient excitation of molecules to high vibrational states and their subsequent dissociation. The conditions for the optimal isotope-selective population of the 2ν3 and 3ν3 states of 235UF6 and 238UF6 molecules were discussed in [19].
4 CONCLUSIONS
A method has been proposed to increase the efficiency of the isotope-selective infrared laser dissociation of 235UF6 molecules under nonequilibrium thermodynamic shock conditions. The method is based on the selective excitation of the \(2{{\nu }_{3}}\) and \(3{{\nu }_{3}}\) vibrational states in 235UF6 molecules by the bichromatic radiation of two CF4 or para-H2 lasers using SF6 molecules that serve as a sensitizer resonantly absorbing the radiation of these lasers. Schemes and parameters for resonant two- and three-photon bichromatic excitation of the \(2{{\nu }_{3}}\) and \(3{{\nu }_{3}}\) states in 235UF6 molecules, which are cooled in a gas-dynamic flow to a temperature of \(T \leqslant 100\) K, by the radiation of the mentioned lasers are given. The results can be applied when using laser infrared dissociation of molecules to separate isotopes.
Change history
19 January 2023
An Erratum to this paper has been published: https://doi.org/10.1134/S0021364022380027
REFERENCES
G. N. Makarov, Phys. Usp. 58, 670 (2015).
G. N. Makarov, Phys. Usp. 65 (2022, in press).
J. W. Eerkens and J. Kim, AIChE J. 56, 2331 (2010).
G. N. Makarov and A. N. Petin, JETP Lett. 97, 76 (2013).
P. Mathi, V. Parthasarathy, A. K. Nayak, J. P. Mittal, and S. K. Sarkar, Proc. Natl. Acad. Sci. India, Sect. A 1, 1 (2015). https://doi.org/10.1007/s40010-015-0249-6
E. Ronander, H. J. Strydom, and R. L. Botha, Prama-J. Phys. 82, 49 (2014).
C. D. Ferguson and J. Boureston, https://www.iranwatch.org/sites/default/files/perspexfwi-Laser.pdf.
K. A. Lyakhov and A. N. Pechen, Appl. Phys. B 126 (8), 141 (2020).
J. Guo, Y.-J. Li, J.-P. Ma, X. Tang, and X.-S. Liu, Chem. Phys. Lett. 773, 138572 (2021).
V. M. Apatin, V. N. Lokhman, G. N. Makarov, N.‑D. D. Ogurok, and E. A. Ryabov, Quantum Electron. 48, 157 (2018).
V. M. Apatin, G. N. Makarov, N.-D. D. Ogurok, A. N. Petin, and E. A. Ryabov, J. Exp. Theor. Phys. 127, 244 (2018).
V. N. Lokhman, G. N. Makarov, A. L. Malinovskii, A. N. Petin, D. G. Poydashev, and E. A. Ryabov, Laser Phys. 28, 105703 (2018).
G. N. Makarov, Phys. Usp. 63, 245 (2020).
V. N. Lokhman, G. N. Makarov, A. N. Petin, D. G. Poydashev, and E. A. Ryabov, J. Exp. Theor. Phys. 128, 188 (2019).
G. N. Makarov and A. N. Petin, JETP Lett. 111, 325 (2020).
G. N. Makarov and A. N. Petin, JETP Lett. 112, 213 (2020).
G. N. Makarov and A. N. Petin, J. Exp. Theor. Phys. 132, 233 (2021).
G. N. Makarov, Quantum Electron. 51, 643 (2021).
G. N. Makarov, J. Exp. Theor. Phys. 133, 669 (2021).
SILEX Process. https://www.chemeurope.com/en/encyclopedia/Silex_Process.html.
SILEX Uranium Enrichment, SILEX Annual Report 2019. http://www.silex.com.au.
G. N. Makarov and A. N. Petin, JETP Lett. 115, 256 (2022).
G. N. Makarov, Phys. Usp. 46, 889 (2003).
G. N. Makarov and A. N. Petin, Quantum Electron. 46, 248 (2016).
J. P. Aldridge, E. G. Brock, H. Filip, et al., J. Chem. Phys. 83, 34 (1985).
R. J. Jensen, P. Judd O’Dean, and J. A. Sullivan, Los Alamos Science 4, 2 (1982).
J. J. Tiee and C. Wittig, Appl. Phys. Lett. 30, 420 (1977).
J. J. Tiee, T. A. Fischer, and C. Wittig, Rev. Sci. Instrum. 50, 958 (1979).
R. L. Byer, IEEE J. Quantum Electron. 12, 732 (1976).
W. R. Trutna and R. L. Byer, Appl. Opt. 19, 301 (1980).
G. A. Laguna, K. C. Kim, C. W. Patterson, M. J. Raisfeld, and D. M. Seitz, Chem. Phys. Lett. 75, 357 (1980).
J. W. Eerkens, R. P. Griot, J. H. Hardin, and R. G. Smith, in Proceedings of the Conference on Lasers and Electro-Optics (Opt. Soc. Am., 1986). https://www.osapublishing.org/abstract.cfm?URI=CLEO-1986-TUI4.
B.-y. Xu, Y. Liu, W.-b. Dong, Ch.-f. Zheng, Y.-y. Zhao, G.-yh. Wang, and H.-sh. Qian, Int. Nucl. Inform. Syst. 21 (20) (1990). https://inis.iaea.org/search/search.aspx?orig_q=RN:21077879.
O. V. Budilova, A. A. Ionin, I. O. Kinyaevskiy, Yu. M. Klimachev, A. A. Kotkov, and A. Yu. Kozlov, Opt. Commun. 345, 163 (2015).
I. Y. Baranov and A. V. Koptev, Proc. SPIE 7915, 7915F (2011). https://doi.org/10.1117/12871578
S. S. Alimpiev, S. M. Nikiforov, B. G. Sartakov, E. M. Khokhlov, and A. L. Shtarkov, Sov. J. Quantum Electron. 15, 289 (1985).
V. M. Apatin, V. N. Lokhman, and G. N. Makarov, Laser Chem. 5, 231 (1985).
Y. Okada, S. Tanimura, H. Okamura, A. Suda, Y. Tashiro, and R. Takeuchi, J. Mol. Struct. 410–411, 299 (1997).
W. B. Person and K. C. Kim, J. Chem. Phys. 69, 1764 (1978).
K. C. Kim, W. B. Person, D. Seitz, and B. J. Krohn, J. Mol. Spectrosc. 76, 322 (1979).
R. S. Karve, S. K. Sarkar, K. V. S. Rama Rao, and J. P. Mittal, Appl. Phys. B 53, 108 (1991).
K. Kim, R. S. McDowell, and W. T. King, J. Chem. Phys. 73, 36 (1980).
D. Hildenbrand, J. Chem. Phys. 66, 4478 (1977).
S. W. Benson, Chem. Rev. 78, 23 (1978).
J. B. Anderson, in Gasdynamics, Molecular Beams and Low Density Gasdynamics, Ed. by P. P. Wegener (Marcel Dekker, New York, 1974).
Ya. B. Zel’dovich and Yu. P. Raizer, Physics of Shock Waves and High-Temperature Hydrodynamic Phenomena (2nd ed., Nauka, Moscow, 1966; Academic, New York, 1966, 1967).
E. V. Stupochenko, S. A. Losev, and A. I. Osipov, Relaxation in Shock Waves (Nauka, Moscow, 1965; Springer, Berlin, 1967).
J. I. Steinfeld, I. Burak, D. G. Sutton, and A. V. Nowak, J. Chem. Phys. 52, 5421 (1970).
H. E. Bass, F. D. Shields, W. D. Breshears, and L. B. Asprey, J. Chem. Phys. 67, 1136 (1977).
V. S. Letokhov and V. P. Chebotaev, Nonlinear Laser Spectroscopy (Nauka, Moscow, 1975; Springer, Berlin, 1977).
C. W. Patterson, B. J. Krohn, and S. N. Pine, Opt. Lett. 6, 39 (1981).
B. J. Krohn, R. S. McDowell, C. W. Patterson, N. G. Nereson, M. J. Raisfield, and K. C. Kim, J. Mol. Spectrosc. 132, 285 (1988).
V. Boudon, G. Pierre, and H. Burger, J. Mol. Spectrosc. 205, 304 (2001).
B. H. Mahan, J. Chem. Phys. 46, 98 (1967).
R. S. McDowell, C. W. Patterson, C. R. Jones, M. I. Buchwald, and J. M. Telle, Opt. Lett. 4, 274 (1979).
V. M. Apatin, V. N. Lokhman, and G. N. Makarov, Opt. Spectrosc. 63, 452 (1987).
ACKNOWLEDGMENTS
I am grateful to A.N. Petin for the help with the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interest.
Additional information
Translated by R. Tyapaev
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makarov, G.N. On the Possibility of the Effective Isotope-Selective Infrared Dissociation of 235UF6 Molecules Vibrationally Excited by Bichromatic Laser Radiation. Jetp Lett. 115, 660–666 (2022). https://doi.org/10.1134/S0021364022600768
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0021364022600768