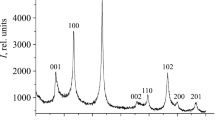
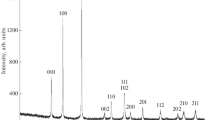
Abstract—
We have studied reaction between mechanochemically preactivated NbCl5 and NaBH4 powders in the molar ratio 1 : 2.4 at temperatures from 873 to 1073 K and reaction times from 8 to 15 h under an argon pressure of 4 MPa in ionic melts (Na2B4O7, КСl, KBr,, and 50 mol % NaCl + 50 mol % KCl or 58 mol % LiCl + 42 mol % KCl eutectic mixture). The results demonstrate that the use of the ionic melts makes it possible to obtain almost spherical niobium diboride nanoparticles with an average size from ~12 to 17 nm (depending on reaction temperature), hexagonal structure (sp. gr. P6/mmm), and unit-cell parameters a = 0.3105–0.3125 nm and c = 0.3269–0.3294 nm.


Similar content being viewed by others
REFERENCES
Serebryakova, T.I., Neronov, V.A., and Peshev, P.D., Vysokotemperaturnye boridy (High-Temperature Borides), Chelyabinsk: Metallurgiya, 1991.
Carenco, S., Portehault, D., Boissiere, C., Mezailles, N., and Sanchez, C., Nanoscaled metal borides and phosphides: recent developments and perspectives, Chem. Rev., 2013, vol. 113, no. 10, pp. 7981–8065. https://doi.org/10.1021/cr400020d
Andrievskii, R.A. and Spivak, I.I., Prochnost’ tugoplavkikh soedinenii i materialov na ikh osnove. Spravochnik (Strength of Refractory Compounds and Related Materials: A Handbook), Chelyabinsk: Metallurgiya, 1989.
Prokhorov, A.M., Lyakishev, N.P., Burkhanov, G.S., and Dement’ev, V.A., High-purity transition-metal borides: promising materials for present-day technology, Inorg. Mater., 1996, vol. 32, no. 11, pp. 1195–1201.
Andrievski, R.A. and Khatchoyan, A.V., Nanomaterials in Extreme Environments, Fundamentals and Applications, Berlin: Springer, 2016. https://doi.org/10.1007/978-3-319-25331-2
Matsudaira, T., Itoh, H., and Naka, S., Synthesis of niobium boride powder by solid-state reaction between niobium and amorphous boron, J. Less-Common Met., 1989, vol. 155, no. 2, pp. 207–214. https://doi.org/10.1016/0022-5088(89)90229-4
Kravchenko, S.E., Vinokurov, A.A., Dremova, N.N., Nadkhina, S.E., and Shilkin, S.P., Synthesis of niobium diboride nanoparticles by the reaction of amorphous boron with niobium in KCl and Na2B4O7 ionic melts, Russ. J. Gen. Chem., 2021, vol. 91, no. 2, pp. 302–304. https://doi.org/10.1134/S1070363221020195
Kravchenko, S.E., Kovalev, D.Yu., Vinokurov, A.A., Dremova, N.N., Ivanov, A.V., and Shilkin, S.P., Synthesis and thermal oxidation stability of nanocrystalline niobium diboride, Inorg. Mater., 2021, vol. 57, no. 10, pp. 1005–1014. https://doi.org/10.1134/S002016852110006X
Peshev, P., Leyarovska, L., and Bliznakov, G., On the borothermic preparation of some vanadium, niobium and tantalum borides, J. Less-Common Met., 1968, vol. 15, pp. 259–267. https://doi.org/10.1016/0022-5088(68)90184-7
Jha, M., Ramanujachary, K.V., Lofland, S.T., Gupta, G., and Ganguli, P.K., Novel borothermal process for the synthesis of nanocrystalline oxides and borides of niobium, J. Dalton Trans., 2011, vol. 40, pp. 7879–7888. https://doi.org/10.1039/c1dt10468c
Maeda, H., Yoshikawa, T., Kusakabe, K., and Morooka, S., Synthesis of ultrafine NbB2 powder by rapid carbothermal reduction in a vertical tubular reactor, J. Alloys Compd., 1994, vol. 215, pp. 127–334. https://doi.org/10.1016/0925-8388(94)90829-X
Gai, P., Yang, Z., Shi, L., Chen, L., Zhao, A., Gu, Y., and Qian, Y., Low temperature synthesis of NbB2 nanorods by a solid-state reaction route, Mater. Lett., 2005, vol. 59, pp. 3550–3552. https://doi.org/10.1016/j.matlet.2005.07.051
Ma, J., Du, Y., Wu, M., Li, G., Feng, Z., Guo, M., Sun, Y., Song, W., Lin, M., and Guo, X., A simple inorganic-solvent route to nanocrystalline niobium diboride, J. Alloys Compd., 2009, vol. 468, pp. 473–476. https://doi.org/10.1016/j.jallcom.2008.01.021
Portehaut, D., Devis, S., Beaunier, P., Gervais, C., Giordano, C., Sanchez, C., and Antonietti, M., A general solution route toward metal boride nanocrystals, Angew. Chem., 2011, vol. 50, pp. 3262–3265. https://doi.org/10.1002/ange.201006810
Jothi, P.R., Yubuta, K., and Fokwa, B.P.T., A simple, general synthetic route toward nanoscale transition metal borides, Adv. Mater., 2018, vol. 30, no. 14, paper 1704181. https://doi.org/10.1002/adma.201704181
Jafari, M., Tajizadegan, H., Golabgir, M.H., Chami, A., and Torabio, O., Investigation on mechanochemical behavior of Al/Mg–B2O3–Nb system reactive mixtures to synthesize niobium diboride, J. Refract. Met. Hard Mater., 2015, vol. 50, pp. 86–92. https://doi.org/10.1016/j.ijrmhm.2014.10.017
Balci, Ö., Aĝaoĝullari, D., Övecoĝlu, M.L., and Duman, I., Synthesis of niobium borides by powder metallurgy methods using Nb2O5, B2O3 and Mg blends, Trans. Nonferrous Met. Soc. China, 2016, vol. 26, pp. 747–758. https://doi.org/10.1016/S1003-6326(16)64165-1
Motojima, S., Sugiyama, K., and Takahashi, Y., Chemical vapor deposition of niobium diboride (NbB2), J. Cryst. Growth, 1975, vol. 30, pp. 233–239. https://doi.org/10.1016/0022-0248(75)90094-9
Gupta, A., Singhal, V., and Pandey, O.P., Facile in-situ synthesis of NbB2 nanoparticles at low temperature, J. Alloys Compd., 2018, vol. 736, pp. 306–313. https://doi.org/10.1016/j.jallcom.2017.10.257
Kravchenko, S.E., Torbov, V.I., and Shilkin, S.P., Nanosized zirconium diboride: synthesis and properties, Russ. J. Inorg. Chem., 2011, vol. 56, no. 4, pp. 506–509. https://doi.org/10.1134/S0036023611040164
Andrievski, R.A., Kravchenko, S.E., and Shilkin, S.P., Some properties of ultrafine zirconium boride powders and films, Jpn. J. Appl. Phys., 1994, vol. 10, pp. 198–199.
Cheng, Y., Choi, S., and Watanabe, T., Synthesis of niobium boride nanoparticle by rf thermal plasma, J. Phys.: Conf. Ser., 2013, vol. 441, paper 012031. https://doi.org/10.1088/1742-6596/441/1/012031
Chen, B., Yang, L., Heng, H., Chen, J., Zhang, L., Xu, L., Qian, Y., and Yang, J., Additive-assisted synthesis of boride, carbide and nitride micro/nanocrystals, J. Solid State Chem., 2012, vol. 194, pp. 219–224. https://doi.org/10.1016/j.jssc.2012.05.032
Vinokurov, A.A., Dremova, N.N., Nadkhina, S.E., Ivanov, A.V., and Shilkin, S.P., Formation of niobium diboride nanoparticles by the reaction of niobium pentachloride with sodium borohydride in ionic melts of alkali metal halides, Russ. J. Gen. Chem., 2022, vol. 92, no. 2, pp. 272–275. https://doi.org/10.1134/S1070363222020189
Fokin, V.N., Fokina, E.E., and Shilkin, S.P., Synthesis of coarsely crystalline metal hydrides, Russ. J. Gen. Chem., 1996, vol. 66, no. 8, pp. 1210–1212.
Semenenko, K.N., Shilkin, S.P., Burnasheva, V.V., Volkova, L.S., and Mozgina, N.G., Reactions of some intermetallic compounds between rare-earth and iron-group metals with nitrogen in the presence of hydrogen, Zh. Obshch. Khim., 1987, vol. 57, no. 4, pp. 729–732.
Bolgar, A.S., Serbova, M.I., Fesenko, V.V., Serebryakova, T.I., and Isaeva, L.P., High-temperature enthalpy and heat capacity of niobium diboride, Teplofiz. Vys. Temp., 1980, vol. 18, no. 6, pp. 1180–1183.
Burgess, D.R., Jr. Thermochemical data in NIST Chemistry WebBook, NIST Standard Reference Database no. 69, Linstrom P.J. and Mallard W.G., Eds., Gaithersburg: National Inst of Standards and Technology. https://doi.org/10.18434/T4D303
Dymova, T.N., Eliseeva, N.G., and Mikheeva, V.I., Thermoanalytical study of sodium borohydride and related substances, Zh. Neorg. Khim., 1967, vol. 12, no. 9, pp. 2317–2320.
Diagrammy sostoyaniya dvoinykh metallicheskikh sistem: Spravochnik (Phase Diagrams of Binary Metallic Systems: A Handbook), Lyakishev, N.P., Ed., Moscow: Mashinostroenie, 1996, vol. 1.
ACKNOWLEDGMENTS
In this study, we used equipment at the Shared Analytical Facilities Center, Institute of Problems of Chemical Physics, Russian Academy of Sciences, and at the Shared Research Facilities Center, Merzhanov Institute of Structural Macrokinetics and Materials Science, Russian Academy of Sciences.
Funding
This work was supported by the Russian Federation Ministry of Science and Higher Education, state research targets for the Institute of Problems of Chemical Physics, Russian Academy of Sciences (state registration no. AAAA-A19-119061890019-5) and the Merzhanov Institute of Structural Macrokinetics and Materials Science, Russian Academy of Sciences (theme no. FFSZ-2022-0009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Vinokurov, A.A., Kovalev, D.Y., Nigmatullina, G.R. et al. Reaction of Niobium Pentachloride with Sodium Borohydride in Ionic Melts. Inorg Mater 58, 838–844 (2022). https://doi.org/10.1134/S0020168522080143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168522080143