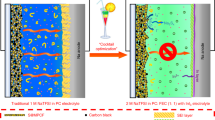
Abstract—
This review examines research reported in the past decade in the field of the fabrication of batteries based on the sodium–sulfur system, capable of operating at an ambient temperature (room-temperature sodium–sulfur (Na–S) batteries). Such batteries differ from currently widespread lithium-ion or lithium–sulfur analogs in that their starting materials are cheaper and more readily available. One of the key problems to be resolved on the way to room-temperature Na–S batteries with high energy density and long-term cycling stability is transport of cell reaction products (sodium polysulfides) to the opposite electrode, which leads to an appreciable battery self-discharge and loss of the active material as a result of redox reactions that are not accompanied by energy generation. This review is aimed at examining approaches to improving the electrochemical performance of the room-temperature Na–S batteries. Particular attention is paid to potential applications of cation-exchange materials capable of suppressing polysulfide anion transport, with a high sodium cation transport rate retained.



Similar content being viewed by others
REFERENCES
Kulova, T.L. and Skundin, A.M., From lithium-ion to sodium-ion battery, Russ. Chem. Bull., 2017, vol. 66, no. 8, pp. 1329–1335.https://doi.org/10.1007/s11172-017-1896-3
Manthiram, A. and Yu, X.W., Ambient temperature sodium–sulfur batteries, Small, 2015, vol. 11, no. 18, pp. 2108–2114.https://doi.org/10.1002/smll.201403257
Morachevskiy, A.G., Popovich, A.A., and Demidov, A.I., New trends of sodium-sulfur batteries development, Peter Great St. Petersburg Polytech. Univ. J. Eng. Sci. Technol., 2017, vol. 23, no. 4, pp. 110–117.https://doi.org/10.18721/JEST.230410
Wang, Y.-X., Zhang, B., Lai, W., Xu, Y., Chou, S.-L., Liu, H.-K., and Dou, S.-X., Room-temperature sodium–sulfur batteries: a comprehensive review on research progress and cell chemistry, Adv. Energy Mater., 2017, vol. 7, no. 24, paper 1602829.https://doi.org/10.1002/aenm.201602829
Zhou, D., Chen, Y., Li, B., Fan, H., Cheng, F., Shanmukaraj, D., Rojo, T., Armand, M., and Wang, G., A stable quasi-solid-state sodium–sulfur battery, Angew. Chem., Int. Ed. Engl., 2018, vol. 57, no. 32, pp. 10168–10172.https://doi.org/10.1002/anie.201805008
Li, T.X., Xu, J., Wang, C.Y., Wu, W.J., Su, D.W., and Wang, G.X., The latest advances in the critical factors (positive electrode, electrolytes, separators) for sodium–sulfur battery, J. Alloys Compd., 2019, vol. 792, pp. 797–817.https://doi.org/10.1016/j.jallcom.2019.03.343
Syali, M.S., Kumar, D., Mishra, K., and Kanchan, D.K., Recent advances in electrolytes for room-temperature sodium–sulfur batteries: a review, Energy Storage Mater., 2020, vol. 31, pp. 352–372.https://doi.org/10.1016/j.ensm.2020.06.023
Yu, X.W. and Manthiram, A., A progress report on metal–sulfur batteries, Adv. Funct. Mater., 2020, vol. 30, no. 39, paper 2004084.https://doi.org/10.1002/adfm.202004084
Wang, Y.J., Zhang, Y.J., Cheng, H.Y., Ni, Z.C., Wang, Y., Xia, G.H., Li, X., and Zeng, X.Y., Research progress toward room temperature sodium sulfur batteries: a review, Molecules, 2021, vol. 26, no. 6, paper 1535.https://doi.org/10.3390/molecules26061535
Branco, H., Castro, R., and Setas Lopes, A., Battery energy storage systems as a way to integrate renewable energy in small isolated power systems, Energy Sustain. Dev., 2018, vol. 43, pp. 90–99.https://doi.org/10.1016/j.esd.2018.01.003
Hu, H., Xie, N., Fang, D., and Zhang, X., The role of renewable energy consumption and commercial services trade in carbon dioxide reduction: evidence from 25 developing countries, Appl. Energy, 2018, vol. 211, pp. 12229–1244.https://doi.org/10.1016/j.apenergy.2017.12.019
Skundin, A.M., Kulova, T.L., and Yaroslavtsev, A.B., Sodium-ion batteries (a review), Russ. J. Electrochem., 2018, vol. 54, no. 2, pp. 113–152.https://doi.org/10.1134/s1023193518020076
Tang, W.W., Aslam, M.K., and Xu, M.W., Towards high performance room temperature sodium–sulfur batteries: strategies to avoid shuttle effect, J. Colloid Interface Sci., 2022, vol. 606, pp. 22–37.https://doi.org/10.1016/j.jcis.2021.07.114
https://periodictable.com/Properties/A/CrustAbundance.an.html.
Yu, X. and Manthiram, A., Capacity enhancement and discharge mechanisms of room-temperature sodium–sulfur batteries, ChemElectroChem, 2014, vol. 1, no. 8, pp. 1275–1280.https://doi.org/10.1002/celc.201402112
Chung, S.H. and Manthiram, A., Current status and future prospects of metal–sulfur batteries, Adv. Mater., 2019, vol. 31, no. 27, paper 1901125.https://doi.org/10.1002/adma.201901125
Park, C.-W., Ryu, H.-S., Kim, K.-W., Ahn, J.-H., Lee, J.-Y., and Ahn, H.-J., Discharge properties of all-solid sodium–sulfur battery using poly(ethylene oxide) electrolyte, J. Power Sources, 2007, vol. 165, no. 1, pp. 450–454.https://doi.org/10.1016/j.jpowsour.2006.11.083
Zhou, D., Tang, X., Guo, X., Li, P., Shanmukaraj, D., Liu, H., Gao, X., Wang, Y., Rojo, T., Armand, M., and Wang, G., Polyolefin-based Janus separator for rechargeable sodium batteries, Angew. Chem., Int. Ed. Engl., 2020, vol. 59, no. 38, pp. 16725–16734.https://doi.org/10.1002/anie.202007008
Kim, I., Kim, C., Kim, H., Kim, K.W., Ahn, J.H., and Ahn, H.J., Initial discharge behavior of an ultra high loading 3D sulfur cathode for a room-temperature Na/S battery, J. Nanosci. Nanotechnol., 2018, vol. 18, no. 9, pp. 6524–6527.https://doi.org/10.1166/jnn.2018.15678
Yu, X.W. and Manthiram, A., Capacity enhancement and discharge mechanisms of room-temperature sodium–sulfur batteries, ChemElectroChem, 2014, vol. 1, no. 8, pp. 1275–1280.https://doi.org/10.1002/celc.201402112
Kim, I., Park, J.Y., Kim, C., Park, J.W., Ahn, J.P., Ahn, J.H., Kim, K.W., and Ahn, H.J., Sodium polysulfides during charge/discharge of the room-temperature Na/S battery using TEGDME electrolyte, J. Electrochem. Soc., 2016, vol. 163, no. 5, pp. A611–A616.https://doi.org/10.1149/2.0201605jes
Kim, I., Park, J.-Y., Kim, C.H., Park, J.-W., Ahn, J.-P., Ahn, J.-H., Kim, K.-W., and Ahn, H.-J., A room temperature Na/S battery using a β″ alumina solid electrolyte separator, tetraethylene glycol dimethyl ether electrolyte, and a S/C composite cathode, J. Power Sources, 2016, vol. 301, pp. 332–337.https://doi.org/10.1016/j.jpowsour.2015.09.120
Paris, J. and Plichon, V., Electrochemical reduction of sulfur in dimethylacetamide, Electrochim. Acta, 1981, vol. 26, no. 12, pp. 1823–1829.https://doi.org/10.1016/0013-4686(81)85170-5
Kim, B.S. and Park, S.M., In situ spectroelectrochemical studies on the reduction of sulfur in dimethyl sulfoxide solutions, J. Electrochem. Soc., 1993, vol. 140, no. 1, pp. 115–122.https://doi.org/10.1149/1.2056070
Gaillard, F., Levillain, E., and Lelieur, J.P., Polysulfides in dimethylformamide: only the radical anions \({\text{S}}_{3}^{ - }\) and \({\text{S}}_{4}^{ - }\) are reducible, J. Electroanal. Chem., 1997, vol. 432, nos. 1–2, pp. 129–138.https://doi.org/10.1016/s0022-0728(97)00192-710.1016/s0022-0728(97)00192-7
Gaillard, F. and Levillain, E., Visible time-resolved spectroelectrochemistry – application to study of the reduction of sulfur (S8) in dimethylformamide, J. Electroanal. Chem., 1995, vol. 398, nos. 1–2, pp. 77–87.https://doi.org/10.1016/0022-0728(95)04144-1
Ryu, H., Kim, T., Kim, K., Ahn, J.H., Nam, T., Wang, G., and Ahn, H.J., Discharge reaction mechanism of room-temperature sodium–sulfur battery with tetra ethylene glycol dimethyl ether liquid electrolyte, J. Power Sources, 2011, vol. 196, no. 11, pp. 5186–5190.https://doi.org/10.1016/j.jpowsour.2011.01.109
Qiang, Z., Chen, Y.M., Xia, Y.F., Liang, W.F., Zhu, Y., and Vogt, B.D., Ultra-long cycle life, low-cost room temperature sodium–sulfur batteries enabled by highly doped (N,S) nanoporous carbons, Nano Energy, 2017, vol. 32, pp. 59–66.https://doi.org/10.1016/j.nanoen.2016.12.018
Wang, Y.X., Yang, J.P., Lai, W.H., Chou, S.L., Gu, Q.F., Liu, H.K., Zhao, D.Y., and Dou, S.X., Achieving high-performance room-temperature sodium sulfur batteries with S@interconnected mesoporous carbon hollow nanospheres, J. Am. Chem. Soc., 2016, vol. 138, no. 51, pp. 16576–16579.https://doi.org/10.1021/jacs.6b08685
Adelhelm, P., Hartmann, P., Bender, C.L., Busche, M., Eufinger, C., and Janek, J., From lithium to sodium: cell chemistry of room temperature sodium–air and sodium–sulfur batteries, Beilstein J. Nanotechnol., 2015, vol. 6, pp. 1016–1055.https://doi.org/10.3762/bjnano.6.105
Guo, Q.B., Li, S., Liu, X.J., Lu, H.C., Chang, X.Q., Zhang, H.S., Zhu, X.H., Xia, Q.Y., Yan, C.L., and Xia, H., Ultrastable sodium–sulfur batteries without polysulfides formation using slit ultramicropore carbon carrier, Adv. Sci., 2020, vol. 7, no. 11, paper 1903246.https://doi.org/10.1002/advs.201903246
Kumar, D., Kanchan, D.K., Kumar, S., and Mishra, K., Recent trends on tailoring cathodes for room-temperature Na–S batteries, Mater. Sci. Technol., 2019, vol. 2, no. 1, pp. 117–129.https://doi.org/10.1016/j.mset.2018.11.007
Yaroslavtsev, A.B. and Stenina, I.A., Carbon coating of electrode materials for lithium-ion batteries, Surf. Innov., 2021, vol. 9, nos. 2–3, pp. 92–110.https://doi.org/10.1680/jsuin.20.00044
Wenzel, S., Metelmann, H., Raiß, c., Dürr, A.K., Janek, J., and Adelhelm, P., Thermodynamics and cell chemistry of room temperature sodium/sulfur cells with liquid and liquid/solid electrolyte, J. Power Sources, 2013, vol. 243, 758–765.https://doi.org/10.1016/j.jpowsour.2013.05.194
Kim, J.S., Ahn, H.J., Kim, I.P., Kim, K.W., Ahn, J.H., Park, C.W., and Ryu, H.S., The short-term cycling properties of Na/PVDF/S battery at ambient temperature, J. Solid State Electrochem., 2008, vol. 12, nos. 7–8, pp. 861–865.https://doi.org/10.1007/s10008-008-0504-8
Liu, D.Z., Li, Z., Li, X., Cheng, Z.X., Yuan, L.X., and Huang, Y.H., Recent advances in cathode materials for room-temperature sodium–sulfur batteries, ChemPhysChem, 2019, vol. 20, no. 23, pp. 3164–3176.https://doi.org/10.1002/cphc.201900595
Carter, R., Oakes, L., Douglas, A., Muralidharan, N., Cohn, A.P., and Pint, C.L., A sugar-derived room-temperature sodium sulfur battery with long term cycling stability, Nano Lett., 2017, vol. 17, no. 3, pp. 1863–1869.https://doi.org/10.1021/acs.nanolett.6b05172
Hu, L., Lu, Y., Zhang, T.W., Huang, T., Zhu, Y.C., and Qian, Y.T., Ultramicroporous carbon through an activation-free approach for Li–S and Na–S batteries in carbonate-based electrolyte, ACS Appl. Mater. Interfaces, 2017, vol. 9, no. 16, pp. 13813–13818.https://doi.org/10.1021/acsami.7b01387
Wei, S., Xu, S., Agrawral, A., Choudhury, S., Lu, Y., Tu, Z., Ma, L., and Archer, L.A., A stable room-temperature sodium–sulfur battery, Nat. Commun., 2016, vol. 7, paper 11722.https://doi.org/10.1038/ncomms11722
Du, W.Y., Wu, Y.K., Yang, T.T., Guo, B.S., Liu, D.Y., Bao, S.J., and Xu, M.W., Rational construction of rGA/VO2 nanoflowers as sulfur multifunctional hosts for room temperature Na–S batteries, Chem. Eng. J., 2020, vol. 379, paper 122359.https://doi.org/10.1016/j.cej.2019.122359
Xin, S., Gu, L., Zhao, N.H., Yin, Y.X., Zhou, L.J., Guo, Y.G., and Wan, L.J., Smaller sulfur molecules promise better lithium–sulfur batteries, J. Am. Chem. Soc., 2012, vol. 134, no. 45, pp. 18510–18513.https://doi.org/10.1021/ja308170k
Guo, Q.B., Sun, S., Kim, K.I., Zhang, H.S., Liu, X.J., Yan, C.L., and Xia, H., A novel one-step reaction sodium–sulfur battery with high areal sulfur loading on hierarchical porous carbon fiber, Carbon Energy, 2021, vol. 3, no. 3, pp. 440–448.https://doi.org/10.1002/cey2.86
Oakes, L., Carter, R., and Pint, C.L., Nanoscale defect engineering of lithium–sulfur battery composite cathodes for improved performance, Nanoscale, 2016, vol. 8, no. 46, pp. 19368–19375.https://doi.org/10.1039/c6nr06332b
Xiao, F.P., Yang, X.M., Wang, H.K., Xu, J., Liu, Y.L., Yu, D.Y.W., and Rogach, A.L., Covalent encapsulation of sulfur in a MOF-derived S, N-doped porous carbon host realized via the vapor-infiltration method results in enhanced sodium–sulfur battery performance, Adv. Energy Mater., 2020, vol. 10, no. 23, paper 2000931.https://doi.org/10.1002/aenm.202000931
Xin, S., Yin, Y.X., Guo, Y.G., and Wan, L.J., A high-energy room-temperature sodium–sulfur battery, Adv. Mater., 2014, vol. 26, no. 8, pp. 1261–1265.https://doi.org/10.1002/adma.201304126
Wei, S.Y., Xu, S.M., Agrawral, A., Choudhury, S., Lu, Y.Y., Tu, Z.Y., Ma, L., and Archer, L.A., A stable room-temperature sodium–sulfur battery, Nat. Commun., 2016, vol. 7, paper 11722.https://doi.org/10.1038/ncomms11722
Wu, H.B., Wei, S.Y., Zhang, L., Xu, R., Hng, H.H., and Lou, X.W., Embedding sulfur in MOF-derived microporous carbon polyhedrons for lithium–sulfur batteries, Chem. – Eur. J., 2013, vol. 19, no. 33, pp. 10804–10808.https://doi.org/10.1002/chem.201301689
Chen, Y.M., Liang, W.F., Li, S., Zou, F., Bhaway, S.M., Qiang, Z., Gao, M., Vogt, B.D., and Zhu, Y., A nitrogen doped carbonized metal-organic framework for high stability room temperature sodium–sulfur batteries, J. Mater. Chem. A, 2016, vol. 4, no. 32, pp. 12471–12478.https://doi.org/10.1039/c6ta04529d
Mou, J.R., Liu, T., Li, Y.J., Zhang, W.J., Li, M., Xu, Y.T., Huang, J.L., and Liu, M.L., Hierarchical porous carbon sheets for high-performance room temperature sodium–sulfur batteries: integration of nitrogen-self-doping and space confinement, J. Mater. Chem. A, 2020, vol. 8, no. 46, pp. 24590–24597.https://doi.org/10.1039/d0ta08876e
Zhang, B.W., Liu, Y.D., Wang, Y.X., Zhang, L., Chen, M.Z., Lai, W.H., Chou, S.L., Liu, H.K., and Dou, S.X., In situ grown S nanosheets on Cu foam: an ultrahigh electroactive cathode for room-temperature Na–S batteries, ACS Appl. Mater. Interfaces, 2017, vol. 9, no. 29, pp. 24446–24450.https://doi.org/10.1021/acsami.7b07615
Zhang, B.W., Sheng, T., Wang, Y.X., Chou, S.L., Davey, K., Dou, S.X., and Qiao, S.Z., Long-life room-temperature sodium–sulfur batteries by virtue of transition-metal-nanocluster–sulfur interactions, Angew. Chem., Int. Ed. Engl., 2019, vol. 58, no. 5, pp. 1484–1488.https://doi.org/10.1002/anie.201811080
Ghosh, A., Kumar, A., Dos, T., Chakrabory, S., Kar, M., Macfarlane, D.R., and Mitra, S., Lewis acid–base interactions between polysulfides and boehmite enables stable room-temperature sodium–sulfur batteries, Adv. Funct. Mater., 2020, vol. 30, no. 50, paper 2005669.https://doi.org/10.1002/adfm.202005669
Mou, J.R., Li, Y.J., Liu, T., Zhang, W.J., Li, M., Xu, Y.T., Zhong, L., Pan, W.H., Yang, C.H., Huang, J.L., and Liu, M.L., Metal-organic frameworks-derived nitrogen-doped porous carbon nanocubes with embedded co nanoparticles as efficient sulfur immobilizers for room temperature sodium–sulfur batteries, Small Methods, 2021, vol. 5, no. 8, paper 2100455.https://doi.org/10.1002/smtd.202100455
Yang, H.L., Zhou, S., Zhang, B.W., Chu, S.Q., Guo, H.P., Gu, Q.F., Liu, H.W., Lei, Y.J., Konstantinov, K., Wang, Y.X., Chou, S.L., Liu, H.K., and Dou, S.X., Architecting freestanding sulfur cathodes for superior room-temperature Na–S batteries, Adv. Funct. Mater., 2021, vol. 31, no. 32, paper 2102280.https://doi.org/10.1002/adfm.202102280
Zhou, J.H., Xu, S.M., and Yang, Y., Strategies for polysulfide immobilization in sulfur cathodes for room-temperature sodium–sulfur batteries, Small, 2021, vol. 17, no. 32, paper 2100057.https://doi.org/10.1002/smll.202100057
Ghosh, A., Shukla, S., Monisha, M., Kumar, A., Lochab, B., and Mitra, S., Sulfur copolymer: a new cathode structure for room-temperature sodium–sulfur batteries, ACS Energy Lett., 2017, vol. 2, no. 10, pp. 2478–2485.https://doi.org/10.1021/acsenergylett.7b00714
Fan, L., Ma, R.F., Yang, Y.H., Chen, S.H., and Lu, B.A., Covalent sulfur for advanced room temperature sodium–sulfur batteries, Nano Energy, 2016, vol. 28, pp. 304–310.https://doi.org/10.1016/j.nanoen.2016.08.056
Wang, J.L., Yang, J., Nuli, Y., and Holze, R., Room temperature Na/S batteries with sulfur composite cathode materials, Electrochem. Commun., 2007, vol. 9, no. 1, pp. 31–34.https://doi.org/10.1016/j.elecom.2006.08.029
Hwang, T.H., Jung, D.S., Kim, J.S., Kim, B.G., and Choi, J.W., One-dimensional carbon–sulfur composite fibers for Na–S rechargeable batteries operating at room temperature, Nano Lett., 2013, vol. 13, no. 9, pp. 4532–4538.https://doi.org/10.1021/nl402513x
Kim, I., Kim, C.H., Choi, S.H., Ahn, J.P., Ahn, J.H., Kim, K.W., Cairns, E.J., and Ahn, H.J., A singular flexible cathode for room temperature sodium/sulfur battery, J. Power Sources, 2016, vol. 307, pp. 31–37.https://doi.org/10.1016/j.jpowsour.2015.12.035
Zhu, T.C., Dong, X.L., Liu, Y., Wang, Y.G., Wang, C.X., and Xia, Y.Y., An all-solid-state sodium–sulfur battery using a sulfur/carbonized polyacrylonitrile composite cathode, ACS Appl. Energy Mater., 2019, vol. 2, no. 7, pp. 5263–5271.https://doi.org/10.1021/acsaem.9b00953
Seong, M., Kim, H., Kim, C., Lim, H.S., Yoon, D.K., Kim, T.H., and Ahn, H.J., Fabrication and electrochemical characterization of sulfurized-polyacrylonitrile nanofiber electrodes for Na/S batteries using various polyacrylonitrile solutions, J. Nanosci. Nanotechnol., 2020, vol. 20, no. 11, pp. 7092–7095.https://doi.org/10.1166/jnn.2020.18828
Murugan, S., Niesen, S., Kappler, J., Kuster, K., Starke, U., and Buchmeiser, M.R., Ultra-stable cycling of high capacity room temperature sodium–sulfur batteries based on sulfurated poly(acrylonitrile), Batteries Supercaps, 2021, vol. 4, no. 10, pp. 1636–1646.https://doi.org/10.1002/batt.202100125
Kim, H., Sadan, M.K., Kim, C., Jo, J., Seong, M., Cho, K.-K., Kim, K.-W., Ahn, J.-H., and Ahn, H.-J., Enhanced reversible capacity of sulfurized polyacrylonitrile cathode for room-temperature Na/S batteries by electrochemical activation, Chem. Eng. J., 2021, vol. 426, paper 130787.https://doi.org/10.1016/j.cej.2021.130787
Zhang, L.L., Zhang, W.H., Zhu, Z.Y., Huang, Q.Q., Liu, X.X., Zhang, M.C., Pei, W.B., and Wu, J.S., Multi-channel sulfurized polyacrylonitrile with hollow structure as cathode for room temperature sodium–sulfur batteries, J. Solid State Chem., 2021, vol. 301, paper 122359.https://doi.org/10.1016/j.jssc.2021.122359
Yu, X. and Manthiram, A., Room-temperature sodium–sulfur batteries with liquid-phase sodium polysulfide catholytes and binder-free multiwall carbon nanotube fabric electrodes, J. Phys. Chem. C, 2014, vol. 118, no. 40, pp. 22952–22959.https://doi.org/10.1021/jp507655u
Ceylan Cengiz, E., Erdol, Z., Sakar, B., Aslan, A., Ata, A., Ozturk, O., and Demir-Cakan, R., Investigation of the effect of using Al2O3–Nafion barrier on room-temperature Na–S batteries, J. Phys. Chem. C, 2017, vol. 121, no. 28, pp. 15120–15126.https://doi.org/10.1021/acs.jpcc.7b04711
Kumar, A., Ghosh, A., Roy, A., Panda, M.R., Forsyth, M., Macfarlane, D.R., and Mitra, S, High-energy density room temperature sodium–sulfur battery enabled by sodium polysulfide catholyte and carbon cloth current collector decorated with MnO2 nanoarrays, Energy Storage Mater., 2019, vol. 20, pp. 196–202.https://doi.org/10.1016/j.ensm.2018.11.031
Yu, X.W. and Manthiram, A., Na2S–carbon nanotube fabric electrodes for room-temperature sodium–sulfur batteries, Chem. – Eur. J., 2015, vol. 21, no. 11, pp. 4233–4237.https://doi.org/10.1002/chem.201405344
Wang, C.L., Wang, H., Hu, X.F., Matios, E., Luo, J.M., Zhang, Y.W., Lu, X., and Li, W.Y., Frogspawn-coral-like hollow sodium sulfide nanostructured cathode for high-rate performance sodium–sulfur batteries, Adv. Energy Mater., 2019, vol. 9, no. 5, paper 1803251.https://doi.org/10.1002/aenm.201803251
Lee, J., Kim, J., Kim, S., and Jo, C., A review on recent approaches for designing the SEI layer on sodium metal anodes, Mater. Adv., 2020, vol. 1, no. 9, pp. 3143–3166.https://doi.org/10.1039/d0ma00695e
Eng, A.Y.S., Kumar, V., Zhang, Y.W., Luo, J.M., Wang, W.Y., Sun, Y.M., Li, W.Y., and Seh, Z.W., Room-temperature sodium–sulfur batteries and beyond: realizing practical high energy systems through anode, cathode, and electrolyte engineering, Adv. Energy Mater., 2021, vol. 11, no. 14, paper 2003493.https://doi.org/10.1002/aenm.202003493
Seh, Z.W., Sun, J., Sun, Y., and Cui, Y., A highly reversible room-temperature sodium metal anode, ACS Cent. Sci., 2015, vol. 1, no. 8, pp. 449–455.https://doi.org/10.1021/acscentsci.5b00328
Wei, S., Choudhury, S., Xu, J., Nath, P., Tu, Z., and Archer, L.A., Highly stable sodium batteries enabled by functional ionic polymer membranes, Adv. Mater., 2017, vol. 29, no. 12, paper 1605512.https://doi.org/10.1002/adma.201605512
Zhao, X., Zhu, Q., Xu, S., Chen, L., Zuo, Z., Wang, X.-M., Liu, S., and Zhang, D., Fluoroethylene carbonate as an additive in a carbonates-based electrolyte for enhancing the specific capacity of room-temperature sodium–sulfur cell, J. Electroanal. Chem., 2019, vol. 832, pp. 392–398.https://doi.org/10.1016/j.jelechem.2018.11.021
Zhang, B.W., Liu, Y.D., Wang, Y.X., Zhang, L., Chen, M.Z., Lai, W.H., Chou, S.L., Liu, H.K., and Dou, S.X., In situ grown S nanosheets on Cu foam: an ultrahigh electroactive cathode for room-temperature Na–S batteries, ACS Appl. Mater. Interfaces, 2017, vol. 9, no. 29, pp. 24446–24450.https://doi.org/10.1021/acsami.7b07615
Komaba, S., Ishikawa, T., Yabuuchi, N., Murata, W., Ito, A., and Ohsawa, Y., Fluorinated ethylene carbonate as electrolyte additive for rechargeable Na batteries, ACS Appl. Mater. Interfaces, 2011, vol. 3, no. 11, pp. 4165–4168.https://doi.org/10.1021/am200973k
Wu, J., Liu, J., Lu, Z., Lin, K., Lyu, Y.-Q., Li, B., Ciucci, F., and Kim, J.-K., Non-flammable electrolyte for dendrite-free sodium–sulfur battery, Energy Storage Mater., 2019, vol. 23, pp. 8–16.https://doi.org/10.1016/j.ensm.2019.05.045
Xu, X.F., Zhou, D., Qin, X.Y., Lin, K., Kang, F.Y., Li, B.H., Shanmukaraj, D., Rojo, T., Armand, M., and Wang, G.X., A room-temperature sodium–sulfur battery with high capacity and stable cycling performance, Nat. Commun., 2018, vol. 9, paper 3870.https://doi.org/10.1038/s41467-018-06443-3
Zhao, Y., Goncharova, L.V., Lushington, A., Sun, Q., Yadegari, H., Wang, B.Q., Xiao, W., Li, R.Y., and Sun, X.L., Superior stable and long life sodium metal anodes achieved by atomic layer deposition, Adv. Mater., 2017, vol. 29, no. 18, paper 1606663.https://doi.org/10.1002/adma.201606663
Wei, S.Y., Choudhury, S., Xu, J., Nath, P., Tu, Z.Y., and Archer, L.A., Highly stable sodium batteries enabled by functional ionic polymer membranes, Adv. Mater., 2017, vol. 29, no. 12, paper 1605512.https://doi.org/10.1002/adma.201605512
Zhang, J., Zhang, G.X., Chen, Z.S., Dai, H.L., Hu, Q.M., Liao, S.J., and Sun, S.H., Emerging applications of atomic layer deposition for lithium–sulfur and sodium–sulfur batteries, Energy Storage Mater., 2020, vol. 26, pp. 513–533.https://doi.org/10.1016/j.ensm.2019.11.025
Zhao, Y., Zhang, L., Liu, J., Adair, K., Zhao, F.P., Sun, Y.P., Wu, T.P., Bi, X.X., Amine, K., Lu, J., and Sun, X.L., Atomic/molecular layer deposition for energy storage and conversion, Chem. Soc. Rev., 2021, vol. 50, no. 6, pp. 3889–3956.https://doi.org/10.1039/d0cs00156b
Wang, L., Wang, T., Peng, L., Wang, Y., Zhang, M., Zhou, J., Chen, M., Cao, J., Fei, H., Duan, X., Zhu, J., and Duan, X., The promises, challenges and pathways to room-temperature sodium–sulfur batteries, Natl. Sci. Rev., 2021.https://doi.org/10.1093/nsr/nwab050
Zhao, Y., Goncharova, L.V., Zhang, Q., Kaghazchi, P., Sun, Q., Lushington, A., Wang, B.Q., Li, R.Y., and Sun, X.L., Inorganic–organic coating via molecular layer deposition enables long life sodium metal anode, Nano Lett., 2017, vol. 17, no. 9, pp. 5653–5659.https://doi.org/10.1021/acs.nanolett.7b02464
Kim, Y.J., Lee, H., Noh, H., Lee, J., Kim, S., Ryou, M.H., Lee, Y.M., and Kim, H.T., Enhancing the cycling stability of sodium metal electrodes by building an inorganic–organic composite protective layer, ACS Appl. Mater. Interfaces, 2017, vol. 9, no. 7, pp. 6000–6006.https://doi.org/10.1021/acsami.6b14437
Zhang, D., Li, B., Wang, S., and Yang, S.B., Simultaneous formation of artificial SEI film and 3D host for stable metallic sodium anodes, ACS Appl. Mater. Interfaces, 2017, vol. 9, no. 46, pp. 40265–40272.https://doi.org/10.1021/acsami.7b12568
Wang, H., Wang, C.L., Matios, E., and Li, W.Y., Critical role of ultrathin graphene films with tunable thickness in enabling highly stable sodium metal anodes, Nano Lett., 2017, vol. 17, no. 11, pp. 6808–6815.
Luo, W., Lin, C.F., Zhao, O., Noked, M., Zhang, Y., Rubloff, G.W., and Hu, L.B., Ultrathin surface coating enables the stable sodium metal anode, Adv. Energy Mater., 2017, vol. 7, no. 2, paper 1601526.https://doi.org/10.1021/acs.nanolett.7b03071
Xu, Z.X., Yang, J., Zhang, T., Sun, L.M., Nuli, Y., Wang, J.L., and Hirano, S., Stable Na metal anode enabled by a reinforced multistructural SEI layer, Adv. Funct. Mater., 2019, vol. 29, no. 27, paper 1901924.https://doi.org/10.1002/adfm.201901924
Lee, D.-J., Park, J.-W., Hasa, I., Sun, Y.-K., Scrosati, B., and Hassoun, J., Alternative materials for sodium ion–sulphur batteries, J. Mater. Chem. A, 2013, vol. 1, no. 17, pp. 5256–5261.https://doi.org/10.1039/c3ta10241f
Yue, J., Han, F.D., Fan, X.L., Zhu, X.Y., Ma, Z.H., Yang, J., and Wang, C.S., High-performance all-inorganic solid-state sodium–sulfur battery, ACS Nano, 2017, vol. 11, no. 5, pp. 4885–4891.https://doi.org/10.1021/acsnano.7b01445
Wang, A.X., Hu, X.F., Tang, H.Q., Zhang, C.Y., Liu, S., Yang, Y.W., Yang, Q.H., and Luo, J.Y., Processable and moldable sodium-metal anodes, Angew. Chem., Int. Ed., 2017, vol. 56, no. 39, pp. 11921–11926.https://doi.org/10.1002/anie.201703937
Fang, Y.J., Luan, D.Y., Chen, Y., Gao, S.Y., and Lou, X.W., Rationally designed three-layered Cu2S@carbon@MoS2 hierarchical nanoboxes for efficient sodium storage, Angew. Chem., Int. Ed., 2020, vol. 59, no. 18, pp. 7178–7183.https://doi.org/10.1002/anie.201915917
Voropaeva, D.Yu., Novikova, S.A., and Yaroslavtsev, A.B., Polymer electrolytes for metal-ion batteries, Russ. Chem. Rev., 2020, vol. 89, no. 10, pp. 1132–1155.https://doi.org/10.1070/rcr4956
Gao, J., Lowe, M.A., Kiya, Y., and Abruña, H.D., Effects of liquid electrolytes on the charge–discharge performance of rechargeable lithium/sulfur batteries: electrochemical and in-situ X-ray absorption spectroscopic studies, J. Phys. Chem. C, 2011, vol. 115, no. 50, pp. 25132–25137.https://doi.org/10.1021/jp207714c
Yu, X. and Manthiram, A., Performance enhancement and mechanistic studies of room-temperature sodium–sulfur batteries with a carbon-coated functional Nafion separator and a Na2S/activated carbon nanofiber cathode, Chem. Mater., 2016, vol. 28, no. 3, pp. 896–905.https://doi.org/10.1021/acs.chemmater.5b04588
Kolositsyn, B.C., Karaseva, E.V., Syng, D.Yu., and Cho, M.D., Cycling a sulfur electrode in mixed electrolytes based on sulfolane: Effect of ethers, Russ. J. Electrochem., 2002, vol. 38, no. 12, pp. 1452–1456.https://doi.org/10.1023/a:1021668721859
Yu, X. and Manthiram, A., A progress report on metal–sulfur batteries, Adv. Funct. Mater., 2020, vol. 30, no. 39, paper 2004084.https://doi.org/10.1002/adfm.202004084
Yim, T., Park, M.-S., Yu, J.-S., Kim, K.J., Im, K.Y., Kim, J.-H., Jeong, G., Jo, Y.-N., Woo, S.-G., Kang, K.-S., Lee, I., and Kim, Y.-J., Effect of chemical reactivity of polysulfide toward carbonate-based electrolyte on the electrochemical performance of Li–S batteries, Electrochim. Acta, 2013, vol. 107, pp. 454–460.https://doi.org/10.1016/j.electacta.2013.06.039
Hayashi, A., Noi, K., Sakuda, A., and Tatsumisago, M., Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries, Nat. Commun., 2012, vol. 3, paper 856.https://doi.org/10.1038/ncomms1843
Nagata, H. and Chikusa, Y., An all-solid-state sodium–sulfur battery operating at room temperature using a high-sulfur-content positive composite electrode, Chem. Lett., 2014, vol. 43, no. 8, pp. 1333–1334.https://doi.org/10.1246/cl.140353
Tanibata, N., Deguchi, M., Hayashi, A., and Tatsumisago, M., All-solid-state Na/S batteries with a Na3PS4 electrolyte operating at room temperature, Chem. Mater., 2017, vol. 29, no. 12, pp. 5232–5238.https://doi.org/10.1021/acs.chemmater.7b01116
An, T., Jia, H.H., Peng, L.F., and Xie, J., Material and interfacial modification toward a stable room-temperature solid-state Na–S battery, ACS Appl. Mater. Interfaces, 2020, vol. 12, no. 18, pp. 20563–20569.https://doi.org/10.1021/acsami.0c03899
Song, S.F., Duong, H.M., Korsunsky, A.M., Hu, N., and Lu, L., A Na+ superionic conductor for room-temperature sodium batteries, Sci. Rep., 2016, vol. 6, paper 32330.https://doi.org/10.1038/srep32330
Oh, J.A.S., He, L.C., Plewa, A., Morita, M., Zhao, Y., Sakamoto, T., Song, X., Zhai, W., Zeng, K.Y., and Lu, L., Composite NASICON (Na3Zr2Si2PO12) solid-state electrolyte with enhanced Na+ ionic conductivity: effect of liquid phase sintering, ACS Appl. Mater. Interfaces, 2019, vol. 11, no. 43, pp. 40125–40133.https://doi.org/10.1021/acsami.9b14986
Yu, X.W. and Manthiram, A., Sodium–sulfur batteries with a polymer-coated NASICON-type sodium-ion solid electrolyte, Matter, 2019, vol. 1, no. 2, pp. 439–451.https://doi.org/10.1016/j.matt.2019.03.008
Armand, M.B., Polymer electrolytes, Ann. Rev. Mater. Sci., 1986, vol. 16, no. 1, pp. 245–261.https://doi.org/10.1146/annurev.ms.16.080186.001333
Yaroslavtseva, T.V., Reznitskikh, O.G., Sherstobitova, E.A., Erkabaev, A.M., Brezhestovsky, M.S., and Bushkova, O.V., Solid polymer electrolytes in a poly(butadiene-acrylonitrile)–LiBr system, Ionics, 2017, vol. 23, no. 12, pp. 3347–3363.https://doi.org/10.1007/s11581-017-2149-z
Zhang, Q.Q., Liu, K., Ding, F., and Liu, X.J., Recent advances in solid polymer electrolytes for lithium batteries, Nano Res., 2017, vol. 10, no. 12, pp. 4139–4174.https://doi.org/10.1007/s12274-017-1763-4
Zhou, D., Shanmukaraj, D., Tkacheva, A., Armand, M., and Wang, G.X., Polymer electrolytes for lithium-based batteries: advances and prospects, Chem, 2019, vol. 5, no. 9, pp. 2326–2352.https://doi.org/10.1016/j.chempr.2019.05.009
Bushkova, O.V., Animitsa, I.E., Lirova, B.I., and Zhukovsky, V.M., Lithium conducting solid polymer electrolytes based on polyacrylonitrile copolymers: ion solvation and transport properties, Ionics, 1997, vol. 3, nos. 5–6, pp. 396–404.https://doi.org/10.1007/bf02375716
Ge, Z., Li, J., and Liu, J., Enhanced electrochemical performance of all-solid-state sodium-sulfur batteries by PEO–NaCF3SO3–MIL-53(al) solid electrolyte, Ionics, 2020, vol. 26, no. 4, pp. 1787–1795.https://doi.org/10.1007/s11581-020-03513-9
Park, C.W., Ahn, J.H., Ryu, H.S., Kim, K.W., and Ahn, H.J., Room-temperature solid-state sodium/sulfur battery, Electrochem. Solid State Lett., 2006, vol. 9, no. 3, pp. A123–A125.https://doi.org/10.1149/1.2164607
Yarmolenko, O.V., Yudina, A.V., Evshchik, E.Yu., Chernyak, A.V., Marinin, A.A., Volkov, V.I., and Kulova, T.L., New network-gel-electrolytes consisting of polyethylene glycol diacrylate, LiBF4, and 1-butyl-3-methylimidazolium tetrafluoroborate, added with alkylene carbonates: The ion transfer mechanism and properties, Russ. J. Electrochem., 2015, vol. 51, no. 5, pp. 421–428.https://doi.org/10.1134/s1023193515050183
Mauger, A., Julien, C.M., Paolella, A., Armand, M., and Zaghib, K., Building better batteries in the solid state: a review, Materials, 2019, vol. 12, no. 23, paper 3892.https://doi.org/10.3390/ma12233892
Kumar, D. and Kanchan, D.K., Dielectric and electrochemical studies on carbonate free Na-ion conducting electrolytes for sodium–sulfur batteries, J. Energy Storage, 2019, vol. 22, pp. 44–49.https://doi.org/10.1016/j.est.2019.01.020
Qiao, L.X., Judez, X., Rojo, T., Armand, M., and Zhang, H., Polymer electrolytes for sodium batteries, J. Electrochem. Soc., 2020, vol. 167, no. 7, paper 070534.https://doi.org/10.1149/1945-7111/ab7aa0
Lonchakova, O.V., Semenikhin, O.A., Zakharkin, M.V., Karpushkin, E.A., Sergeyev, V.G., and Antipov, E.V., Efficient gel-polymer electrolyte for sodium-ion batteries based on poly(acrylonitrile-co-methyl acrylate), Electrochim. Acta, 2020, vol. 334, paper 135512.https://doi.org/10.1016/j.electacta.2019.135512
Lim, D.H., Agostini, M., Ahn, J.H., and Matic, A., An electrospun nanofiber membrane as gel-based electrolyte for room-temperature sodium–sulfur batteries, Energy Technol., 2018, vol. 6, no. 7, pp. 1214–1219.https://doi.org/10.1002/ente.201800170
Kumar, D., Suleman, M., and Hashmi, S.A., Studies on poly(vinylidene fluoride-co-hexafluoropropylene) based gel electrolyte nanocomposite for sodium–sulfur batteries, Solid State Ionics, 2011, vol. 202, no. 1, pp. 45–53.https://doi.org/10.1016/j.ssi.2011.09.001
Singh, R., Maheshwaran, C., Kanchan, D.K., Mishra, K., Singh, P.K., and Kumar, D., Ion-transport behavior in tetraethylene glycol dimethyl ether incorporated sodium ion conducting polymer gel electrolyte membranes intended for sodium battery application, J. Mol. Liq., 2021, vol. 336, paper 116594.https://doi.org/10.1016/j.molliq.2021.116594
Syali, M.S., Mishra, K., Kanchan, D.K., and Kumar, D., Studies on a novel Na+ superionic conducting polymer gel cocktail electrolyte membrane immobilizing molecular liquid mixture of carbonates, tetraglyme and ionic liquid, J. Mol. Liq., 2021, vol. 341, paper 116922.https://doi.org/10.1016/j.molliq.2021.116922
Eshetu, G.G., Armand, M., Scrosati, B., and Passerini, S., Energy storage materials synthesized from ionic liquids, Angew. Chem., Int. Ed., 2014, vol. 53, no. 49, pp. 13342–13359.https://doi.org/10.1002/anie.201405910
Eshetu, G.G., Elia, G.A., Armand, M., Forsyth, M., Komaba, S., Rojo, T., and Passerini, S., Electrolytes and interphases in sodium-based rechargeable batteries: recent advances and perspectives, Adv. Energy Mater., 2020, vol. 10, no. 20, paper 2000093.https://doi.org/10.1002/aenm.202000093
Zhang, H., Li, C.M., Piszcz, M., Coya, E., Rojo, T., Rodriguez-Martinez, L.M., Armand, M., and Zhou, Z.B., Single lithium-ion conducting solid polymer electrolytes: advances and perspectives, Chem. Soc. Rev., 2017, vol. 46, no. 3, pp. 797–815.https://doi.org/10.1039/c6cs00491a
Di Nato, V., Bettiol, M., Bassetto, F., Boaretto, N., Negro, E., Lavina, S., and Bertasi, F., Hybrid inorganic–organic nanocomposite polymer electrolytes based on Nafion and fluorinated TiO2 for PEMFCs, Int. J. Hydrogen Energy, 2012, vol. 37, no. 7, pp. 6169–6181.https://doi.org/10.1016/j.ijhydene.2011.07.131
Lemay, N., Mikhaylin, S., Mareev, S., Pismenskaya, N., Nikonenko, V., and Bazinet, L., How demineralization duration by electrodialysis under high frequency pulsed electric field can be the same as in continuous current condition and that for better performances?, J. Membr. Sci., 2020, vol. 603, paper 117878.https://doi.org/10.1016/j.memsci.2020.117878
Filippov, S.P. and Yaroslavtsev, A.B., Hydrogen energy: development prospects and materials, Russ. Chem. Rev., 2021, vol. 90, no. 6, pp. 627–643.https://doi.org/10.1070/rcr5014
Achoh, A.R., Zabolotsky, V.I., Lebedev, K.A., Sharafan, M.V., and Yaroslavtsev, A.B., Electrochemical properties and selectivity of bilayer ion-exchange membranes in ternary solutions of strong electrolytes, Membr. Membr. Technol., 2021, vol. 3, no. 1, pp. 52–71.https://doi.org/10.1134/s2517751621010029
Sanginov, E.A., Kayumov, R.R., Shmygleva, L.V., Lesnichaya, V.A., Karelin, A.I., and Dobrovolsky, Y.A., Study of the transport of alkali metal ions in a nonaqueous polymer electrolyte based on Nafion, Solid State Ionics, 2017, vol. 300, pp. 26–31.https://doi.org/10.1016/j.ssi.2016.11.017
Zyubina, T.S., Zyubin, A.S., Dobrovol’skii, Yu.A., and Volokhov, V.M., Nonaqueous LiNafion-based polymeric electrolyte: quantum-chemical modeling, Russ. J. Inorg. Chem., 2017, vol. 62, no. 8, pp. 1051–1057.https://doi.org/10.1134/s0036023617080198
Pan, Q.Y., Li, Z., Zhang, W.C., Zeng, D.L., Sun, Y.B., and Cheng, H.S., Single ion conducting sodium ion batteries enabled by a sodium ion exchanged poly(bis(4-carbonyl benzene sulfonyl)imide-co-2,5-diamino benzesulfonic acid) polymer electrolyte, Solid State Ionics, 2017, vol. 300, pp. 60–66.https://doi.org/10.1016/j.ssi.2016.12.001
Voropaeva, D.Y., Novikova, S.A., Kulova, T.L., and Yaroslavtsev, A.B., Solvation and sodium conductivity of nonaqueous polymer electrolytes based on Nafion-117 membranes and polar aprotic solvents, Solid State Ionics, 2018, vol. 324, pp. 28–32.https://doi.org/10.1016/j.ssi.2018.06.002
Voropaeva, D.Y., Novikova, S.A., Kulova, T.L., and Yaroslavtsev, A.B., Conductivity of Nafion-117 membranes intercalated by polar aprotonic solvents, Ionics, 2018, vol. 24, no. 6, pp. 1685–1692.https://doi.org/10.1007/s11581-017-2333-1
Li, Z., Lu, W.H., Zhang, N., Pan, Q.Y., Chen, Y.Z., Xu, G.D., Zeng, D.L., Zhang, Y.F., Cai, W.W., Yang, M., Yang, Z.H., Sun, Y.B., Ke, H.Z., and Cheng, H.S., Single ion conducting lithium sulfur polymer batteries with improved safety and stability, J. Mater. Chem. A, 2018, vol. 6, no. 29, pp. 14330–14338.https://doi.org/10.1039/c8ta04619k
Voropaeva, D., Novikova, S., Xu, T.W., and Yaroslavtsev, A., Polymer electrolytes for LIBs based on perfluorinated sulfocationic Nepem-117 membrane and aprotic solvents, J. Phys. Chem. B, 2019, vol. 123, no. 48, pp. 10217–10223.https://doi.org/10.1021/acs.jpcb.9b08555
Zyubina, T.S., Sanginov, E.A., Zyubin, A.S., Dobrovol’skii, Yu.A., Volokhov, V.M., Klyucharev, V.V., and Bukun, N.G., Polymeric electrolyte comprising a Nafion membrane plasticized by dimethyl sulfoxide and the transport specifics of alkali metal ions in it: quantum-chemical simulation, Russ. J. Inorg. Chem., 2020, vol. 65, no. 3, pp. 360–372.https://doi.org/10.1134/s0036023620030201
Istomina, A.S., Yaroslavtseva, T.V., Reznitskikh, O.G., Kayumov, R.R., Shmygleva, L.V., Sanginov, E.A., Dobrovolsky, Y.A., and Bushkova, O.V., Li-Nafion membrane plasticised with ethylene carbonate/sulfolane: influence of mixing temperature on the physicochemical properties, Polymers, 2021, vol. 13, no. 7, paper 1150.https://doi.org/10.3390/polym13071150
Sanginov, E.A., Borisevich, S.S., Kayumov, R.R., Istomina, A.S., Evshchik, E.Y., Reznitskikh, O.G., Yaroslavtseva, T.V., Melnikova, T.I., Dobrovolsky, Y.A., and Bushkova, O.V., Lithiated Nafion plasticised by a mixture of ethylene carbonate and sulfolane, Electrochim. Acta, 2021, vol. 373, paper 137914.https://doi.org/10.1016/j.electacta.2021.137914
Golubenko, D.V., Pourcelly, G., and Yaroslavtsev, A.B., Permselectivity and ion-conductivity of grafted cation-exchange membranes based on UV-oxidized polymethylpenten and sulfonated polystyrene, Sep. Purif. Technol., 2018, vol. 207, pp. 329–335.https://doi.org/10.1016/j.seppur.2018.06.041
Berezina, N.P., Kononenko, N.A., Sytcheva, A.A.R., Loza, N.V., Shkirskaya, S.A., Hegman, N., and Pungor, A., Perfluorinated nanocomposite membranes modified by polyaniline: electrotransport phenomena and morphology, Electrochim. Acta, 2009, vol. 54, no. 8, pp. 2342–2352.https://doi.org/10.1016/j.electacta.2008.10.048
Porozhnyy, M., Huguet, P., Cretin, M., Safronova, E., and Nikonenko, V., Mathematical modeling of transport properties of proton-exchange membranes containing immobilized nanoparticles, Int. J. Hydrogen Energy, 2016, vol. 41, no. 34, pp. 15605–15614.https://doi.org/10.1016/j.ijhydene.2016.06.057
Stenina, I.A. and Yaroslavtsev, A.B., Ionic mobility in ion-exchange membranes, Membranes, 2021, vol. 11, no. 3, paper 198.https://doi.org/10.3390/membranes11030198
Porozhnyy, M.V., Shkirskaya, S.A., Butylskii, D.Y., Dotsenko, V.V., Safronova, E.Y., Yaroslavtsev, A.B., Deabate, S., Huguet, P., and Nikonenko, V., Physicochemical and electrochemical characterization of Nafion-type membranes with embedded silica nanoparticles: effect of functionalization, Electrochim. Acta, 2021, vol. 370, paper 137689.https://doi.org/10.1016/j.electacta.2020.137689
Quartarone, E. and Mustarelli, P., Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives, Chem. Soc. Rev., 2011, vol. 40, no. 5, pp. 2525–2540.https://doi.org/10.1039/c0cs00081g
Cheng, X.L., Pan, J., Zhao, Y., Liao, M., and Peng, H.S., Gel polymer electrolytes for electrochemical energy storage, Adv. Energy Mater., 2018, vol. 8, no. 7, paper 1702184.https://doi.org/10.1002/aenm.201702184
Wang, Y., Travas-Sejdic, J., and Steiner, R., Polymer gel electrolyte supported with microporous polyolefin membranes for lithium ion polymer battery, Solid State Ionics, 2002, vol. 148, nos. 3–4, pp. 443–449.https://doi.org/10.1016/s0167-2738(02)00085-1
Stenina, I., Golubenko, D., Nikonenko, V., and Yaroslavtsev, A., Selectivity of transport processes in ion-exchange membranes: relationship with the structure and methods for its improvement, Int. J. Mol. Sci., 2020, vol. 21, no. 15, paper 5517.https://doi.org/10.3390/ijms21155517
Yu, X. and Manthiram, A., Ambient-temperature sodium–sulfur batteries with a sodiated Nafion membrane and a carbon nanofiber-activated carbon composite electrode, Adv. Energy Mater., 2015, vol. 5, no. 12, paper 1500350.https://doi.org/10.1002/aenm.201500350
Bauer, I., Kohl, M., Althues, H., and Kaskel, S., Shuttle suppression in room temperature sodium–sulfur batteries using ion selective polymer membranes, Chem. Commun., 2014, vol. 50, no. 24, pp. 3208–3210.https://doi.org/10.1039/c4cc00161c
Wenzel, S., Leichtweiss, T., Weber, D.A., Sann, J., Zeier, W.G., and Janek, J., Interfacial reactivity benchmarking of the sodium ion conductors Na3PS4 and sodium beta-alumina for protected sodium metal anodes and sodium all-solid-state batteries, ACS Appl. Mater. Interfaces, 2016, vol. 8, no. 41, pp. 28216–28224.https://doi.org/10.1021/acsami.6b10119
Ren, Y.X., Jiang, H.R., Zhao, T.S., Zeng, L., and Xiong, C., Remedies of capacity fading in room-temperature sodium–sulfur batteries, J. Power Sources, 2018, vol. 396, pp. 304–313.https://doi.org/10.1016/j.jpowsour.2018.06.056
Funding
This work was supported by the Russian Federation Ministry of Science and Higher Education as part of the state research target for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Novikova, S.A., Voropaeva, D.Y. & Yaroslavtsev, A.B. Trends in the Development of Room-Temperature Sodium–Sulfur Batteries. Inorg Mater 58, 333–348 (2022). https://doi.org/10.1134/S0020168522040124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168522040124