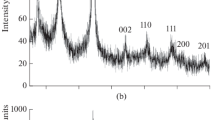
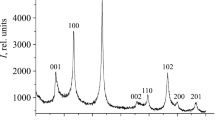
Abstract—
A method has been developed for the synthesis of nanocrystalline NbB2 powder with an average particle size of 65 nm. The material has been prepared by reacting Nb and amorphous B powders (1 : 2 ratio) in Na2B4O7, KCl, and KBr ionic melts after pretreatment with hydrogen and activation in a high-energy planetary mill for 40 min. The synthesis process was run for 32 h at 800°C in argon at a pressure of 4 MPa. The results demonstrate that, independent of the composition and nature of the melt, the process yields NbB2 nanoparticles with hexagonal symmetry (sp. gr. P6/mmm) and unit-cell parameters a = 0.3100–0.3108 nm and c = 0.3278–0.3298 nm. The products of the oxidation of the NbB2 nanoparticles with atmospheric oxygen during heating to 1000°C and isothermal oxidation at 400, 450, 500, 550, and 600°C have been characterized by thermal analysis, X-ray diffraction, scanning electron microscopy, energy dispersive X-ray microanalysis, X-ray photoelectron spectroscopy, and IR frustrated total internal reflection spectroscopy. The rate constants for the oxidation of NbB2 nanoparticles at these temperatures have been determined to be 0.0013, 0.045, 0.47, 2.61, and 8.83 h–1, respectively. The oxidation onset temperature has been determined to be 310°C. The effective activation energy evaluated for the oxidation of NbB2 nanoparticles from the temperature dependence of rate constants is 220 ± 8 kJ/mol.








Similar content being viewed by others
REFERENCES
Serebryakova, T.I., Neronov, V.A., and Peshev, P.D., Vysokotemperaturnye boridy (High-Temperature Borides), Chelyabinsk: Metallurgiya, 1991.
Carenco, S., Portehault, D., Boissiere, C., Mezailles, N., and Sanchez, C., Nanoscaled metal borides and phosphides: recent developments and perspectives, Chem. Rev., 2013, vol. 113, no. 10, pp. 7981–8065.https://doi.org/10.1021/cr400020d
Andrievskii, R.A. and Spivak, I.I., Prochnost’ tugoplavkikh soedinenii i materialov na ikh osnove. Spravochnik (Strength of Refractory Compounds and Related Materials: A Handbook), Chelyabinsk: Metallurgiya, 1989.
Prokhorov, A.M., Lyakishev, N.P., Burkhanov, G.S., and Dement’ev, V.A., High-purity transition-metal borides: promising materials for present-day technology, Inorg. Mater., 1996, vol. 32, no. 11, pp. 1195–1201.
Andrievski, R.A. and Khatchoyan, A.V., Nanomaterials in Extreme Environments, Fundamentals and Applications, Berlin: Springer, 2016.https://doi.org/10.1007/978-3-319-2533-2
Matsudaira, T., Itoh, H., and Naka, S., Synthesis of niobium boride powder by solid-state reaction between niobium and amorphous boron, J. Less-Common Met., 1989, vol. 155, no. 2, pp. 207–214.https://doi.org/10.1016/0022-5088(89)90229-4
Peshev, P., Leyarovska, L., and Bliznakov, G., On the borothermic preparation of some vanadium, niobium and tantalum borides, J. Less-Common Met., 1968, vol. 15, pp. 259–267.https://doi.org/10.1016/0022-5088(68)90184-7
Jha, M., Ramanujachary, K.V., Lofland, S.E., Gupta, G., and Ganguli, A.K., Novel borothermal process for the synthesis of nanocrystalline oxides and borides of niobium, J. Dalton Trans., 2011, vol. 40, pp. 7879–7888.https://doi.org/10.1039/c1dt10468c
Maeda, H., Yoshikawa, T., Kusakabe, K., and Morooka, S., Synthesis of ultrafine NbB2 powder by rapid carbothermal reduction in a vertical tubular reactor, J. Alloys Compd., 1994, vol. 215, pp. 127–334.https://doi.org/10.1016/0925-8388(94)90829-X
Gai, P., Yang, Z., Shi, L., Chen, L., Zhao, A., Gu, Y., and Qian, Y., Low temperature synthesis of NbB2 nanorods by a solid-state reaction route, Mater. Lett., 2005, vol. 59, pp. 3550–3552.https://doi.org/10.1016/j.matlet.2005.07.051
Ma, J., Du, Y., Wu, M., Li, G., Feng, Z., Guo, M., Sun, Y., Song, W., Lin, M., and Guo, X., A simple inorganic-solvent route to nanocrystalline niobium diboride, J. Alloys Compd., 2009, vol. 468, pp. 473–476.https://doi.org/10.1016/j.jallcom.2008.01.021
Jothi, P.R., Yubuta, K., and Fokwa, B.P.T., A simple, general synthetic route toward nanoscale transition metal borides, Adv. Mater., 2018, vol. 30, no. 14, paper 1704181.https://doi.org/10.1002/adma.201704181
Portehaut, D., Devis, S., Beaunier, P., Gervais, C., Giordano, C., Sanchez, C., and Antonietti, M., A general solution route toward metal boride nanocrystals, Angew. Chem., 2011, vol. 50, pp. 3262–3265.https://doi.org/10.1002/ange.201006810
Jafari, M., Tajizadegan, H., Golabgir, M.H., Chami, A., and Torabio, O., Investigation on mechanochemical behavior of Al/Mg–B2O3–Nb system reactive mixtures to synthesize niobium diboride, J. Refract. Met. Hard Mater., 2015, vol. 50, pp. 86–92.https://doi.org/10.1016/j.ijrmhm.2014.10.017
Balci, Ö., Aĝaoĝullari, D., Övecoĝlu, M.L., and Duman, I., Synthesis of niobium borides by powder metallurgy methods using Nb2O5, B2O3 and Mg blends, Trans. Nonferrous Met. Soc. China, 2016, vol. 26, pp. 747–758.https://doi.org/10.1016/S1003-6326(16)64165-1
Motojima, S., Sugiyama, K., and Takahashi, Y., Chemical vapor deposition of niobium diboride (NbB2), J. Cryst. Growth, 1975, vol. 30, pp. 233–239.https://doi.org/10.1016/0022-0248(75)90094-9
Kravchenko, S.E., Torbov, V.I., and Shilkin, S.P., Nanosized zirconium diboride: synthesis and properties, Russ. J. Inorg. Chem., 2011, vol. 56, no. 4, pp. 506–509.https://doi.org/10.1134/S0036023611040164
Gupta, A., Singhal, V., and Pandey, O.P., Facile in-situ synthesis of NbB2 nanoparticles at low temperature, J. Alloys Compd., 2018, vol. 736, pp. 306–313.https://doi.org/10.1016/j.jallcom.2017.10.257
Ilyushchenko, N.G., Anfinogenov, A.I., and Shurov, N.I., Vzaimodeistvie metallov v ionnykh rasplavakh (Interactions between Metals in Ionic Melts), Moscow: Nauka, 1991.
Kravchenko, S.E., Domashnev, I.A., Dremova, N.N., Vinokurov, A.A., and Shilkin, S.P., Synthesis of vanadium diboride nanoparticles via reaction of amorphous boron with vanadium in KCl and Na2B4O7 ionic melts, Inorg. Mater., 2019, vol. 55, no. 5, pp. 443–448.https://doi.org/10.1134/S002016851905011X
Volkova, L.S., Shulga, Yu.M., and Shilkin, S.P., Synthesis of nano-sized titanium diboride in a melt of anhydrous sodium tetraborate, Russ. J. Gen. Chem., 2012, vol. 82, no. 5, pp. 819–821.https://doi.org/10.1134/S1070363212050027
Kravchenko, S.E., Vinokurov, A.A., Dremova, N.N., Nadkhina, S.E., and Shilkin, S.P., Synthesis of niobium diboride nanoparticles by the reaction of amorphous boron with niobium in KCl and Na2B4O7 ionic melts, Russ. J. Gen. Chem., 2021, vol. 91, no. 2, pp. 302–304.https://doi.org/10.1134/S1070363221020195
Fokin, V.N., Fokina, E.E., and Shilkin, S.P., Synthesis of coarsely crystalline metal hydrides, Russ. J. Gen. Chem., 1996, vol. 66, no. 8, pp. 1210–1212.
Fokin, V.N., Fokina, E.E., Tarasov, B.P., and Shilkin, S.P., Synthesis of the tetragonal titanium dihydride in ultradispersed state, Int. J. Hydrogen Energy, 1999, vol. 24, nos. 2–3, pp. 111–114.https://doi.org/10.1016/S0360-3199(98)00070-6
Trusov, B.G., Thermodynamic analysis of high-temperature states and processes and its practical implementation, Doctoral (Eng.) Dissertation, Moscow: Moscow State Tech. Univ., 1984.
Sinyarev, G.B., Vasolin, N.A., Trusov, B.G., and Moiseev, G.K., Primenenie EVM dlya termodinamicheskikh raschetov metallurgicheskikh protsessov (Use of Computers for Thermodynamic Evaluation of Metallurgical Processes), Moscow: Nauka, 1982.
Diagrammy sostoyaniya dvoinykh metallicheskikh sistem: Spravochnik (Phase Diagrams of Binary Metallic Systems: A Handbook), Lyakishev, N.P., Ed., Moscow: Mashinostroenie, 1996, vol. 1.
Bolgar, A.S., Serbova, M.I., Fesenko, V.V., Serebryakova, T.I., and Isaeva, L.P., High-temperature enthalpy and heat capacity of niobium diboride, Teplofiz. Vys. Temp., 1980, vol. 18, no. 6, pp. 1180–1183.
Joyner, D.J. and Hercules, D.M., Chemical bonding and electronic structure of B2O3, H3BO3, and BN: ESCA, Auger, SIMS and SXS study, J. Chem. Phys., 1980, vol. 72, no. 2, pp. 1095–1108.https://doi.org/10.1063/1.439251
Ong, C.W., Huang, H., Zheng, B., Kwok, R.W.M., Hui, Y.Y., and Lau, W.M., X-ray photoemission spectroscopy of nonmetallic materials: electronic structures of boron and BxOy , J. Appl. Phys., 2004, vol. 95, no. 7, pp. 3527–3534.https://doi.org/10.1063/1.1651321
Sidorov, T.A. and Sobolev, N.N., Infrared and Raman spectra of boric anhydride: III. Interpretation of the vibrational spectrum of boric anhydride and calculation of the isotopic effect, Opt. Spektrosk., 1958, vol. 4, no. 1, pp. 9–16.
Bethell, D.E. and Sheppard, N., The infrared spectrum and structure of boric oxide, Trans. Faraday Soc., 1955, vol. 51, pp. 9–15.
ACKNOWLEDGMENTS
In this study, we used equipment at the Shared Analytical Facilities Center, Institute of Problems of Chemical Physics, Russian Academy of Sciences, and at the Shared Research Facilities Center, Merzhanov Institute of Structural Macrokinetics and Materials Science, Russian Academy of Sciences.
Funding
This work was supported by the Russian Federation Ministry of Science and Higher Education, state research targets for the Institute of Problems of Chemical Physics, Russian Academy of Sciences (theme state registration no. AAAA-A19-119061890019-5) and the Merzhanov Institute of Structural Macrokinetics and Materials Science, Russian Academy of Sciences (theme no. 44.1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Kravchenko, S.E., Kovalev, D.Y., Vinokurov, A.A. et al. Synthesis and Thermal Oxidation Stability of Nanocrystalline Niobium Diboride. Inorg Mater 57, 1005–1014 (2021). https://doi.org/10.1134/S002016852110006X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002016852110006X