Abstract—
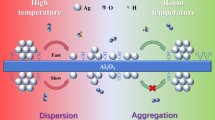
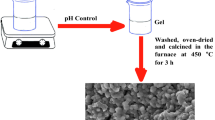
Weakly aggregated, highly dispersed precursor powders with a specific surface area of 350–360 m2/g have been prepared by the citrate sol–gel method in the CеO2〈ZrO2〉–Al2O3 system. Heat treatment of the precursors in the temperature range 700–1000°C led to the formation of powders based on a fluorite-like Ce0.7Zr0.3O2 solid solution with a crystallite size under 30 nm. The presence of alumina has been shown to inhibit the Ce0.7Zr0.3O2 crystallite growth process. Using low-temperature nitrogen adsorption measurements, we have studied texture characteristics of the powders obtained at 1000°C: they have a specific surface area of 90 and 105 m2/g and a specific pore volume of up to 0.380 cm3/g, with a unimodal pore size distribution in the range 2.5–10 nm. We examine the effect of thermal “aging” at a temperature of 1000°C for 50 h on the structure and particle size of the powders. Based on the experimental data obtained, we propose a process for the synthesis of nanocrystalline mesoporous powders in the CеO2〈ZrO2〉–Al2O3 system for the preparation of supports of an active phase (Pt, Pd, or Rh) in three-way catalysts.











Similar content being viewed by others
REFERENCES
Anderson, J.A., Daley, R.A., Christou, S.Y., and Efstathiou, A.M., Regeneration of thermally aged Pt–Rh/CexZr1 – xO2–Al2O3 model three-way catalysts by oxychlorination treatments, Appl. Catal., B, 2006, vol. 64, nos. 3–4, pp. 189–200.https://doi.org/10.1016/j.apcatb.2005.12.007
Ozawa, M., Okouchi, T., and Haneda, M., Three way catalytic activity of thermally degenerated Pt/Al2O3 and Pt/CeO2-ZrO2 modified Al2O3 model catalysts, Catal. Today, 2015, vol. 242, pp. 329–337. https://doi.org/10.1016 /j.cattod.2014.06.013
Monte, R.D., Fornasiero, P., Kašpar, J., Rumori, P., Gubitosa, G., and Graziani, M., Pd/Ce0.6Zr0.4O2/Al2O3 as advanced materials for three-way catalysts: Part 1. Catalyst characterisation, thermal stability and catalytic activity in the reduction of NO by CO, Appl. Catal., B, 2000, vol. 24, nos. 3–4, pp. 157–167.https://doi.org/10.1016/S0926-3373(99)00102-2
Li, J., Liu, X., and Zhan, W., Preparation of high oxygen storage capacity and thermally stable ceria–zirconia solid solution, Catal. Sci. Technol., 2016, vol. 6, pp. 897–907.https://doi.org/10.1039/C5CY01571E
Priya, N.S., Somayaji, C., and Kanagaraj, S., Optimization of Ce0.6Zr0.4 – xAl1.3xO2 solid solution based on oxygen storage capacity, J. Nanopart. Res., 2014, vol. 16, no. 2, pp. 2214–2215.https://doi.org/10.1007/s11051-013-2214-0
Ivanova, A.I., Physicochemical and catalytic properties of systems based on CeO2, Kinet. Catal., 2009, vol. 50, no. 6, pp. 797–815.https://doi.org/10.1134/S00231584090-60020
Kuznetsova, T.G. and Sadykov, V.A., Specific features of the defect structure of metastable nanodisperse ceria, zirconia and related materials, Kinet. Catal., 2008, vol. 49, no. 6, pp. 840–858.https://doi.org/10.1134/S0023158408060098
Monte, R., Kaspar, J., Bradshaw, H., and Norman, C., Rationale for the development of thermally stable nanostructured CeO2−ZrO2-containing mixed oxides, J. Rare Earths, 2008, vol. 26, no. 2, pp. 136–140.
Ivanov, V.K., Polezhaeva, O.S., Kopitsa, G.P., Fedorov, P.P., Pranzas, K., and Runov, V.V., Specifics of high-temperature coarsening of ceria nanoparticles, Russ. J. Inorg. Chem., 2009, vol. 54, no. 11, pp. 1689–1696.https://doi.org/10.1134/S0036023609-110023
Morozova, L.V., Kalinina, M.V., Arsent’ev, M.Yu., and Shilova, M.V., Influence of cryochemical and ultrasonic processing on the texture and thermal decomposition of xerogels and properties of nanoceramics in the ZrO2〈Y2O3〉–Al2O3 system, Inorg. Mater., 2017, vol. 53, no. 6, pp. 640–647.https://doi.org/10.1134/S0020168517060115
Gusev, A.I., Nanomaterialy, nanostruktury, nanotekhnologii (Nanomaterials, Nanostructures, and Nanotechnologies), Moscow: Nauka–Fizmatlit, 2007, 2nd ed.
Al’myasheva, O.V., Fedorov, B.A., Smirnov, A.V., and Gusarov, V.V., Size, morphology, and structure of zirconium dioxide nanopowder particles prepared under hydrothermal conditions, Nanosist.: Fiz., Khim., Mat., 2010, vol. 1, no. 1, pp. 26–37.
Pechini, M., US Patent, no. 3330697, 1967.
Tai, L.W. and Lessing, P.A., Modified resin-intermediate processing of perovskite powders: Part II. Processing for fine, nonagglomerated Sr-doped lanthanum chromite powders, J. Mater. Res., 1992, no. 7, pp. 511–519.
Worayingyong, A., Worayingyong, A., Kangvansura, P., Ausadasuk, S., and Praserthdam, P., The effect of preparation: Pechini and Schiff base methods, on adsorbed oxygen of LaCO3 perovskite oxidation catalysts, Colloids Surf., A, 2008, vol. 315, nos. 1–3, pp. 217–225.
Tani, E., Yoshimura, M., and Somiy, S., Revised phase diagram of the system ZrO2–CeO2 below 1400°C, J. Am. Ceram. Soc., 1983, vol. 66, no. 7, pp. 506–510.https://doi.org/10.1111/j.1151-2916.1983.tb10591.x
Panova, T.I., Arsent’ev, M.Yu., Morozova, L.V., and Drozdova, I.A., Synthesis and investigation of the structure of ceramic nanopowders in the ZrO2–CeO2–Al2O3 system, Glass Phys. Chem., 2010, vol. 36, no. 4, pp. 470–477.
Morrison, R.T. and Boyd, R.N., Organic Chemistry, Boston: Allyn and Bacon, 1969, 2nd ed.
Gusev, A.I. and Kurlov, A.S., Particle (grain) size characterization of nanocrystalline materials, Metallofiz. Noveishie Tekhnol., 2008, vol. 30, no. 5, pp. 679–694.
Sing, K.S.W., Everett, D.H., and Haul, R.A.W., Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity, Pure Appl. Chem., 1985, vol. 57, pp. 603–619.
Gregg, S.J. and Sing, K.S.W., Adsorption, Surface Area and Porosity, New York.: Academic, 1982.
Simonenko, N.P., Sakharov, K.A., Simonenko, E.P., Sevastyanov, G.V., and Kuznetsov, N.T., Glycol−citrate synthesis of ultrafine lanthanum zirconate, Russ. J. Inorg. Chem., 2015, vol. 60, no. 12, pp. 1452–1458.https://doi.org/10.1134/S003602361512-0232
Korolev, P.V., Knyazev, A.V., Gavrilov, I.R., Gavrilov, M.R., and Korolev, A.V., X-ray diffraction and calorimetric studies of powder nanocrystalline systems based on ZrO2(Y) and Al2O3 with second insoluble component, Phys. Solid State, 2012, vol. 54, no. 2, pp. 267–272.https://doi.org/10.1134/S1063783412020114
Ivanova, A.S., Aluminum oxide and systems based on it: properties and applications, Kinet. Catal., 2012, vol. 53, no. 4, pp. 425–439.https://doi.org/10.1134/S0023158412040039
ACKNOWLEDGMENTS
We are grateful to A.E. Lapshin for doing the X-ray diffraction work and to I.A. Drozdova for performing the electron-microscopic characterizations of the precursor powders.
Funding
This work was supported by the Russian Federation Ministry of Science and Higher Education through the state research target for the Grebenshchikov Institute of Silicate Chemistry, Russian Academy of Sciences, as part of the research theme Inorganic Synthesis and Characterization of Ceramic and Organic–Inorganic Composite Materials and Coatings, state registration (Executive Branch’s Center for Information Technologies and Systems) no. AAAA-A19-119022290091-8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Morozova, L.V. Synthesis of Nanocrystalline Powders in the CеO2〈ZrO2〉–Al2O3 System by the Citrate Sol–Gel Method. Inorg Mater 57, 154–163 (2021). https://doi.org/10.1134/S0020168521020096
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168521020096