Abstract—
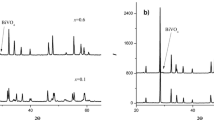
NaxSr1 – 2xNdxMoO4 (x = 0–0.5, Δx = 0.1) solid solutions with the scheelite structure have been synthesized for the first time and their crystallographic parameters have been determined as functions of composition and temperature. Their thermal expansion coefficients have been determined in the temperature range 25–1000°C: αa = (12.9–14.9) × 10–6 °C–1 and αc = (24.9–25.9) × 10–6 °C–1. Using spark plasma sintering at tmax ≈ 872–985°C and τ ≈ 650–750 s, we have prepared ceramics with relative densities in the range 97–99%.






Similar content being viewed by others
REFERENCES
Borisevich, V.D., Yan, J., Smirnov, A.Y., Bonarev, A.K., Zeng, S., Sulaberidze, G.A., and Jiang, D., Cascade design for isotopically modified molybdenum as an alternative to zirconium alloys, Chem. Eng. Res. Des., 2017, vol. 128, pp. 257–264. https://doi.org/10.1016/j.cherd.2017.10.018
Burakov, B.E., Anderson, E.B., Shabalev, S.I., Strykanova, E.E., Ushakov, S.V., Trotabas, M., Blanc, J.-Y., Winter, P., and Duco, J., The behavior of nuclear fuel in first days of the Chernobyl accident, Mater. Res. Soc. Symp. Proc., 1997, pp. 1297–1308. https://doi.org/10.1557/PROC-465-1297
Foreman, M.R.St., An introduction to serious nuclear accident chemistry, Cogent Chem., 2015, vol. 1, no. 1, pp. 1–18. https://doi.org/10.1080/23312009.2015.1049111
Brinkman, K., Fox, K., Marra, J., Reppert, J., Crum, J., and Tang, M., Single phase melt processed powellite (Ba,Ca)MoO4 for the immobilization of Mo-rich nuclear waste, J. Alloys Compd., 2013, vol. 551, pp. 136–142. https://doi.org/10.1016/j.jallcom.2012.09.049
Bosbach, D., Rabung, T., Brandt, F., and Fanghänel, T., Trivalent actinide coprecipitation with powellite (CaMoO4): secondary solid solution formation during HLW borosilicate-glass dissolution, Radiochim. Acta, 2004, vol. 92, pp. 639–643. https://doi.org/10.1524/ract.92.9.639.54976
Morozov, V.A., Mironov, A.V., Lazoryak, B.I., Khaikina, E.G., Basovich, O.M., Rossell, M.D., and Van Tendeloo, G., Ag1/8Pr5/8MoO4: an incommensurately modulated scheelite-type structure, J. Solid State Chem., 2006, vol. 179, no. 4, pp. 1183–1191. https://doi.org/10.1016/j.jssc.2005.12.041
Evdokimov, A.A., Efremov, V.A., Trunov, V.K., Kleinman, I.A., and Dzhurinskii, B.F., Soedineniya redkozemel’nykh elementov. Molibdaty. Vol’framaty (Rare-Earth Molybdates and Tungstates), Moscow: Nauka, 1991.
Hanusa, J., Raman scattering and infra-red spectra of tungstates KLn(WO4)2-Family (Ln: La–Lu), J. Mol. Struct., 1984, vol. 114, pp. 471–474. https://doi.org/10.1016/0022-2860(84)87189-6
Kolysh, A.V., Kryukova, A.I., and Korshunov, I.A., Effect of component solubility on the formation of rare-earth and divalent metal (calcium, strontium, and barium) molybdates, Radiokhimiya, 1973, vol. 15, no. 1, pp. 3–7.
Kolysh, A.V., Kryukova, A.I., and Korshunov, I.A., Effect of the nature of the solvent on the formation of mixed crystals of promethium with alkaline-earth molybdates in a melt: II. Cocrystallization of promethium with calcium molybdate, Radiokhimiya, 1970, vol. 12, no. 6, pp. 814–818.
Kolysh, A.V., Kryukova, A.I., and Korshunov, I.A., Effect of the nature of the solvent on the formation of mixed crystals of promethium with alkaline-earth molybdates in a melt: I. Cocrystallization of promethium with calcium molybdate, Radiokhimiya, 1970, vol. 12, no. 6, pp. 808–814.
Pages, M. and Freundlich, W., Phases of scheelite structure in the neptunium molybdate and sodium or lithium molybdate systems, J. Inorg. Nucl. Chem., 1972, vol. 34, no. 9, pp. 2797–2801. https://doi.org/10.1016/0022-1902(72)80584-0
Lee, M.R. and Mahe, P., Molybdates et tungstates d’uranium IV et de sodium, C. R. Acad. Sci. Paris, 1974, vol. 279, no. 26, pp. 1137–1170.
Tabuteau, A. and Pages, M., Identification and crystal chemistry of double molybdates of alkali metals (K, Rb, Cs) and transuranium elements (Np, Pu, Am), J. Inorg. Nucl. Chem., 1980, vol. 42, no. 3, pp. 401–403. https://doi.org/10.1016/0022-1902(80)80015-7
Tabuteau, A., Pages, M., and Freundlich, W., Sur les phases de structure scheelite dans les systèms molybdate de plutonium–molybdate de lithium ou sodium, Mater. Res. Bull., 1972, vol. 7, no. 7, pp. 691–697. https://doi.org/10.1016/0025-5408(72)90058-X
Basovich, O.M., Khaikina, E.G., Solodovnikov, S.F., and Tsyrenova, G.D., Phase formation in the systems Li2MoO4–K2MoO4–Ln2(MoO4)3 (Ln = La, Nd, Dy, Er) and properties of triple molybdates LiKLn2(MoO4)4, J. Solid State Chem., 2005, vol. 178, no. 5, pp. 1580–1588. https://doi.org/10.1016/j.jssc.2004.12.016
Basovich, O.M., Khaikina, E.G., Vasil’ev, E.V., and Frolov, A.M., Phase formation in Li2MoO4–Rb2MoO4–Ln2(MoO4)3 systems and properties of LiRbLn2(MoO4)4, Zh. Neorg. Khim., 1995, vol. 40, no. 12, pp. 2047–2051.
Morozov, V.A., Lazoryak, B.I., Smirnov, V.A., Mikhailin, V.V., Basovich, O.M., and Khaikina, E.G., Crystal structures and luminescence properties of ternary molybdates LiMNd2(MoO4)4 (M = K, Rb, Tl), Russ. J. Inorg. Chem., 2001, vol. 46, no. 6, pp. 873–879.
Basovich, O.M. and Khaikina, E.G., Synthesis and characterization of lithium thallium rare-earth ternary molybdates, Zh. Neorg. Khim., 1994, vol. 39, no. 9, pp. 1419–1420.
Szillat, H. and Müller-Buschbaum, Hk., Synthese und Kristallstructur von KCuHoMo4O16, Z. Naturforsch., 1994, vol. 49, pp. 350–354.
Müller-Buschbaum, Hk. and Gallinat, St., Synthese und Röntgenstrukturanalyse von KCuGd2Mo4O16 und KCuTb2Mo4O16, Z. Naturforsch., 1995, vol. 50, pp. 1794–1798.
Klevtsova, R.F., Vasil’ev, A.D., Glinskaya, L.A., Kruglik, A.I., Kozhevnikova, N.M., and Korsun, V.P., Crystal structure of the Li3Ba2Ln3(MoO4)8 (Ln = Gd, Tm) ternary molybdates, Zh. Strukt. Khim., 1992, vol. 33, no. 3, pp. 126–130.
Achary, S.N., Patwe, S.J., Mathews, M.D., and Tyagi, A.K., High temperature crystal chemistry and thermal expansion of synthetic powellite (CaMoO4): a high temperature X-ray diffraction (HT-XRD) study, J. Phys. Chem. Solids, 2006, vol. 67, pp. 774–781. https://doi.org/10.1016/j.jpcs.2005.11.009
Binoy, K.M., Hrudananda, J., Asuvathraman, R., and Govindan Kutty, K.V., Electrical conductivity and thermal expansion behavior of MMoO4 (M = Ca, Sr and Ba), J. Alloys Compd., 2015, vol. 640, pp. 475–479. https://doi.org/10.1016/j.jallcom.2015.04.054
Pang, L.-X., Zhou, D., Cai, C.-L., and Liu, W.-G., Infrared spectroscopy and microwave dielectric properties of ultra-low temperature firing (K0.5La0.5)MoO4 ceramics, Mater. Lett., 2013, vol. 92, pp. 36–38. https://doi.org/10.1016/j.matlet.2012.10.082
Pang, L.-X., Zhou, D., Guo, J., Yue, Z.-X., and Yao, X., Microwave dielectric properties of (Li0.5Ln0.5)MoO4 (Ln = Nd, Er, Gd, Y, Yb, Sm, and Ce) ceramics, J. Am. Ceram. Soc., 2015, vol. 98, no. 1, pp. 130–135. https://doi.org/10.1111/jace.13247
Hérisson de Beauvoir, T., Molinari, F., Chung-Seu, U.C., Michau, D., Denux, D., and Josse, M., Densification of MnSO4 ceramics by cool-SPS: evidences for a complex sintering mechanism and magnetoelectric coupling, J. Eur. Ceram. Soc., 2018, vol. 38, no. 11, pp. 3867–3874.https://doi.org/10.1016/j.jeurceramsoc.2018.04.005
Herisson de Beauvoir, T., Sangregorio, A., Bertrand, A., Payen, C., Michau, D., Chunga, U.-C., and Josse, M., Cool-SPS stabilization and sintering of thermally fragile, potentially magnetoelectric, NH4FeP2O7 (CJ-3:IL04), Ceram. Int. (in press). https://doi.org/10.1016/j.ceramint.2018.12.103
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tokarev, M.G., Potanina, E.A., Orlova, A.I. et al. Thermal Expansion of Scheelite-Like Molybdate Powders and Ceramics. Inorg Mater 55, 730–736 (2019). https://doi.org/10.1134/S0020168519070203
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168519070203