Abstract
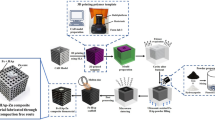
Based on theoretical and experimental analysis of ionic equilibria in buffer solutions, we have found conditions for octacalcium phosphate (OCP), Ca8(HPO4)2(PO4)4 ∙ 5H2O, synthesis. Succinate-substituted OCP, Ca8(HPO4)2–хSucx(PO4)4 ∙ 5H2O, with x = 0.8–0.9 has been synthesized at pH 5.5 and t = 60°C in 3 h via α-TCP hydrolysis in a 0.25 M succinic buffer solution. The synthesis product is more stable to thermolysis than is pure OCP: the apatite-like product is stable up to 630°C. The succinate@OCP powder has been used as a filler for poly(ethylene glycol)diacrylate-based hydrogel in producing a composite implant by stereolithographic 3D printing.
Similar content being viewed by others
References
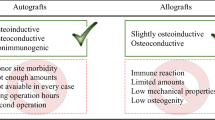
Porter, J.R., Ruckh, T.T., and Popat, K.C., Bone tissue engineering: a review in bone biomimetics and drug delivery strategies, Am. Inst. Chem. Eng. Biotechnol. Prog., 2009, vol. 25, pp. 1539–1560.
Ievlev, V.M., Putlyaev, V.I., Safronova, T.V., and Evdokimov, P.V., Additive technologies for making highly permeable inorganic materials with tailored morphological architectonics for medicine, Inorg. Mater., 2015, vol. 51, no. 13, pp. 1295–1313.
Sivashanmugam, A., Arun Kumar, R., Vishnu Priya, M., Nair, S.V., and Jayakumar, R., An overview of injectable polymeric hydrogels for tissue engineering, Eur. Polym. J., 2015, vol. 72, pp. 543–565.
Suzuki, O., Octacalcium phosphate (OCP)–based bone substitute materials, Jpn. Dental Sci. Rev., 2013, vol. 49, no. 2, pp. 58–71.
Barinov, S.M., Calcium phosphate–based ceramic and composite materials for medical applications, Usp. Khim., 2010, vol. 79, no. 1, pp. 15–32.
Gurin, A.N., Komlev, V.S., Fadeeva, I.N., and Barinov, S.M., Octacalcium phosphate: a precursor for biomineralization and promising osteoplastic material, Stomatologiya, 2010, no. 4, pp. 65–72.
Suzuki, O., Octacalcium phosphate: osteoconductivity and crystal chemistry, Acta Biomater., 2010, vol. 6, no. 9, pp. 3379–3387.
Monma, H. and Goto, M., Complexes of apatitic layered compound Ca8(HPO4)2(PO4)4 ∙ 5H2O with dicarboxylates, J. Inclusion Phenom., 1984, vol. 2, nos. 1–2, pp. 127–134.
Markovic, M., Fowler, B.O., and Brown, W.E., Octacalcium phosphate carboxylates: IV. Kinetics of formation and solubility of octacalcium phosphate succinate, J. Cryst. Growth, 1994, vol. 135, nos. 3–4, pp. 533–538.
Sakamoto, K., Yamaguchi, S., Kaneno, M., Fujihara, I., Satoh, K., and Tsunawaki, Y., Synthesis and thermal decomposition of layered calcium phosphates including carboxylate ions, Thin Solid Films, 2008, vol. 517, no. 4, pp. 1354–1357.
Yokoi, T., Kamitakahara, M., and Ohtsuki, C., Continuous expansion of the interplanar spacing of octacalcium phosphate by incorporation of dicarboxylate ions with a side chain, Dalton Trans., 2015, vol. 44, no. 17, pp. 7943–7950.
Tanaka, H., Watanabe, T., Chikazawa, M., Kandori, K., and Ishikawa, T., Formation and structure of calcium alkyl phosphates, Colloids Surf., A, 1998, vol. 139, no. 3, pp. 341–349.
Tanaka, H. and Ihata, D., Phase transformation of calcium phenyl phosphate in calcium hydroxyapatite using alkaline phosphatase at body temperature, Mater. Res. Bull., 2010, vol. 45, no. 2, pp. 103–108.
Ravoo, B.J. and Engberts, J.B.F.N., Single–tail phosphates containing branched alkyl chains. Synthesis and aggregation in water of a novel class of vesicle–forming surfactants, Langmuir, 1994, vol. 10, no. 14, pp. 1735–1740.
Kukueva, E.V., Putlyaev, V.I., Tikhonov, A.A., and Safronova, T.V., Octacalcium phosphate as a precursor for the synthesis of composite bioceramics, Inorg. Mater., 2017, vol. 53, no. 2, pp. 212–219.
Davis, B.L., “Standardless” X–ray diffraction quantitative analysis, Atmos. Environ. (1967–1989), 1980, vol. 14, no. 2, pp. 217–220.
Raynaud, S., Champion, E., Bernache–Assollant, D., and Laval, J., Determination of calcium/phosphorus atomic ratio of calcium phosphate apatites using X–ray diffractometry, J. Am. Ceram. Soc., 2001, vol. 84, no. 2, pp. 359–366.
Puigdomenech, I., Windows Software for the Graphical Presentation of Chemical Speciation, 219th ACS Natl. Meet., San Francisco: Am. Chem. Soc., 2000, Abstract I&EC–248. www.kth.se/che/medusa/.
Gustafsson, J.P., Visual MINTEQ Version 3.1, Stockholm: Department of Sustainable Development, Environmental Science and Engineering, KTH, 2013. https://vminteq.lwr.kth.se/.
Jacobs, P.F., Rapid Prototyping and Manufacturing: Fundamentals of Stereolithography, Deaborn: Society of Manufacturing Engineers Publishers, 1992.
Boanini, E., Gazzano, M., Rubini, K., and Bigi, A., Collapsed octacalcium phosphate stabilized by ionic substitutions, Cryst. Growth Des., 2010, vol. 10, pp. 3612–3617.
Markoviec, M., Fowler, J.B., and Brown, W.E., Octacalcium phosphate carboxylates. 1. Preparation and identification, Chem. Mater., 1993, vol. 5, no. 10, pp. 1401–1405.
Davies, E., Müller, K.H., Wong, W.C., Pickard, C.J., Reid, D.G., Skepper, J.N., and Duer, M.J., Citrate bridges between mineral platelets in bone, Proc. Natl. Acad. Sci. USA, 2014, vol. 111, no. 14, pp. 1354–1363.
Yokoi, T., Kato, H., Kim., I.Y., Kikuta, K., Kamitakahara, M., Kawashita, M., and Ohtsuki, C., Formation of octacalcium phosphates with Co–incorporated succinate and suberate ions, Dalton Trans., 2012, vol. 41, no. 9, pp. 2732–2737.
Putlyaev, V.I., Kukueva, E.V., Safronova, T.V., Ivanov, V.K., and Churagulov, B.R., Features of octacalcium phosphate thermolysis, Refract. Ind. Ceram., 2014, vol. 54, no. 5, pp. 420–424.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Tikhonov, E.V. Kukueva, P.V. Evdokimov, E.S. Klimashina, V.I. Putlyaev, I.M. Shcherbakov, V.E. Dubrov, 2018, published in Neorganicheskie Materialy, 2018, Vol. 54, No. 10, pp. 1123–1132.
Rights and permissions
About this article
Cite this article
Tikhonov, A.A., Kukueva, E.V., Evdokimov, P.V. et al. Synthesis of Substituted Octacalcium Phosphate for Filling Composite Implants Based on Polymer Hydrogels Produced by Stereolithographic 3D Printing. Inorg Mater 54, 1062–1070 (2018). https://doi.org/10.1134/S0020168518100175
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168518100175