Abstract
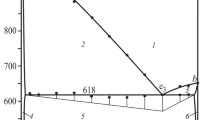
We have developed a procedure for the synthesis of phase-pure α- and β-Cu2V2O7. Thermal analysis and X-ray diffraction demonstrate that the β-phase (monoclinic structure) exists at low temperatures (stability range 25–610°C), while α-Cu2V2O7 (orthorhombic structure) is stable in the range 610–704°C. The α-phase observed during cooling, in particular at room temperature, is in a metastable state. The melting of the high-temperature phase γ-Cu2V2O7, which forms between 704 and 716°C, has the highest rate in the range 770–785°S and is accompanied by peritectic decomposition and oxygen gas release. Subsequent cooling gives rise to four exothermic peaks, one of which (780.9°C) is attributable to the crystallization of the peritectic melt, one (620.1°C) is due to the γ → α → β phase transformations of Cu2V2O7, and the other two arise from the crystallization of multicomponent low-melting-point eutectics containing α- and β-Cu2V2O7, CuVO3, and other compounds.
Similar content being viewed by others
References
Panero, S., Pasquali, M., and Pistoia, G., Rechargeable Li/Li1 + x V3O8 Cells, J. Electrochem. Soc., 1983, vol. 130, no. 5, pp. 1225–1227.
Abraham, K.M., Goldman, J.L., and Dempsey, M.D., Rechargeable Lithium/Vanadium Oxide Cells Utilizing 2MeTHF/LiAsF6, J. Electrochem. Soc., 1981, vol. 128, no. 12, pp. 2493–2501.
Yamaki, J. and Yamaji, A., Layered Materials for Lithium Secondary Batteries, Phys. B + C, 1981, vol. 105, nos. 1–3, pp. 466–470.
Tranchant, A., Messina, R., and Perichon, J., Electrochemical Behaviour of CuO + V2O5 Systems in Molten Dimethyl Sulfone at 150°C, J. Electroanal. Chem., 1988, vol. 242, nos. 1–2, pp. 181–190.
Pommer, J., Kataev, V., Choi, K.-Y., et al., Interplay between Structure and Magnetism in the Spin-Chain Compound (Cu,Zn)2V2O7, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, vol. 67, paper 214 410.
He, Z. and Ueda, Y., Paramagnetic Anisotropy and Spin-Flop Transition in Single Crystals of the Quasi-One-Dimensional System β-Cu2V2O7, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, vol. 77, paper 052 402.
Ponomarenko, L.A., Vasil’ev, A.N., Antipov, E.V., and Velikodny, Y.A., Magnetic Properties of Cu2V2O7, Phys. B (Amsterdam, Neth.), vols. 284–288, pp. 1459–1460.
Clark, G.M. and Garlick, R., Formation and Properties of Copper(II) Divanadate(V), J. Inorg. Nucl. Chem., 1978, vol. 40, no. 7, pp. 1347–1349.
Petrova, S.A., Zakharov, R.G., Rotermel’, M.V., et al., A New High-Temperature Phase of Copper Pyrovana-date, Dokl. Akad. Nauk, 2005, vol. 400, no. 6, pp. 770–773.
Mercurio-Lavaud, D. and Frit, B., Structure crystalline de la variete basse temperature du pyrovanadate de cuivre: Cu2V2O7α, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1973, vol. 29, no. 12, pp. 2737–2741.
Powder Diffraction File PDF2, JCPDS-ICDD, 2006
Mercurio-Lavaud, D. and Frit, B., Crystal Structure of High-Temperature Variety of Copper Pyrovanadate β-Cu2V2O7 // C. R. Acad. Sci., 1973, vol. 277, no. 21, pp. 1101–1104.
Ait Salah, A., Benkhouja, K., Jaafari, K., et al., Structural Characterization and Magnetic Properties of Divanadates ZnMV2O7 (M = Co, Ni and Cu), J. Alloys Compd., 2005, vol. 402, pp. 213–218.
Nord, A.G., Aberg, G., Stefanidis, Th., et al., Preparation and Characterization of Some Divalent-Metal Ortho- and Divanadates, Chem. Scr., 1985, vol. 25, no. 3, pp. 212–216.
Slobodin, B.V. and Kiseleva, N.V., Sistemy M2O-CuO-V2O5, Zh. Neorg. Khim., 1993, vol. 38, no. 7, pp. 1225–1228.
Eguchi, M., Furusawa, I., Miura, T., and Kishi, T., Lithium Insertion Characteristics of β-Cu2V2O7, Solid State Ionics, 1994, vol. 68, nos. 1–2, pp. 159–164.
Carvalho, M.D., Costa, F.M.A., Pereira, J.S., et al., New Preparation Method of Lan + 1NinO3n + 1 − δ (n = 2, 3), J. Mater. Chem., 1997, vol. 7, no. 10, pp. 2107–2111.
Krasnenko, T.I., Zolotukhina, L.V., and Vasyutinskaya, E.V., Thermal Strain in Copper Pyrovanadate, Zh. Neorg. Khim., 2001, vol. 46, no. 7, pp. 1161–1163.
Baklanova, I.V., Zabolotskaya, E.V., Zolotukhina, L.V., Krasnenko, T.I., and Rotermel’, M.V., Investigation of Copper Pyrovanadate near Its α → βPhase Transition, Khimiya tverdogo tela i funktsional’nye materialy: IV seminar SO RAN-UrO RAN (Solid-State Chemistry and Functional Materials: IV Workshop of the Siberian and Ural Divisions of the Russ. Acad. Sci.), Yekaterinburg, 2004, pp. 31.
Rotermel’, M.V., Crystal Chemistry and Phase Equilibria of M2V2O7 (M = Cu, Zn, Cd), Extended Abstract of Cand. Sci (Chem.) Dissertation, Yekaterinburg: Inst. of Solid State Chemistry, Ural. Div., Russ. Acad. Sci., 2005.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © B.V. Slobodin, R.F. Samigullina, 2010, published in Neorganicheskie Materialy, 2010, Vol. 46, No. 2, pp. 236–241.
Rights and permissions
About this article
Cite this article
Slobodin, B.V., Samigullina, R.F. Thermoanalytical study of the polymorphism and melting behavior of Cu2V2O7 . Inorg Mater 46, 196–200 (2010). https://doi.org/10.1134/S0020168510020196
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168510020196