Abstract
Polycondensation of complexes of chloromethanes with triphenylphosphine by the action of low-voltage electric discharges in the liquid phase gives nanosized solid products. The elemental composition involving the generation of element distribution maps (scanning electron microscopy–energy dispersive X‑ray spectroscopy mapping) and the component composition (by direct evolved gas analysis–mass spectrometry) of the solid products have been studied. The elemental and component compositions of the result-ing structures vary widely depending on the chlorine content in the substrate and on the amount of triphenylphosphine taken. Thermal desorption analysis revealed abnormal behavior of HCl and benzene present in the solid products. In thermal desorption spectra, these components appear at an uncharacteristically high temperature. The observed anomaly in the behavior of HCl is due to HCl binding into a complex of the solid anion \({\text{HCl}}_{2}^{ - }\) with triphenyl(chloromethyl)phosphonium chloride, which requires a relatively high temperature (up to 800 K) to decompose. The abnormal behavior of benzene is associated with its encapsulated state in nanostructures. The appearance of benzene begins at 650 K and continues up to temperatures above 1300 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
At the end of the last century, the theory and practice of creating nanostructured materials began to develop rapidly. The progress achieved stimulated the formation of new interdisciplinary directions in chem-istry, physics, materials science, and medicine and the discovery of new allotropic modifications of carbon (fullerenes, carbyne, nanotubes, graphene, etc.) [1–4]. Thus, the addition of carbon nanotubes to a polymer matrix imparts fundamentally new properties to the resulting material due to the unique characteristics of nanotubes. For example, single-walled nanotubes have a Young modulus of 320–1470 GPa [5], and multi-walled nanotubes have a Young modulus of 270–950 GPa [6]. Thus, it becomes possible to create composite materials, based on polymers and carbon nanotube additives, having fundamentally new characteristics that can only be provided by additives of nanostructures.
The main difficulty standing in the way of solving this problem is the need to ensure a good mechanical interface between the nanotube surface and the material. To overcome this difficulty, it is necessary to create a chemical bond between the nanotube surface and the matrix, which requires surface functionalization of nanotubes.
Fullerenes and nanotubes are known to be easily modified with various precursors to obtain decorated nanostructures in which the precursors are immobilized on the surface of nanostructures by chemical bonds. As it turned out, this does not exhaust the transformative abilities of nanostructures. It was found that fullerenes and nanotubes can react not only with analogs and various precursors, but also with each other to result in unique symbiotic structures, such as nanotubes that locate fullerenes inside them. These structures were obtained by laser evaporation of graphite with metal catalysts [7]. Nanopeapods are hybrid systems consisting of a carbon nanotube (pod) with molecules or atoms (peas) located in its cavity. Instead of fullerenes, other substances in different phase states can also be encapsulated inside nanotubes [8–10]. An example is the recent discovery of the phenomenon of self-organization of water molecules, placed inside a carbon nanotube of 1.05 nm in diameter, into chains and their freezing at a system temperature of 130–138°C [11–13]. Such self-organization and retention of substances inside nanotubes with their alignment in a chain apparently has a common basis with the formation of peapods.
Modification of nanostructures with the formation of specified functional groups is an effective method of involving these compounds in the practical field and the fabrication of new materials with specified properties [14, 15]. Modification of carbon structures with the inclusion of heteroatoms directly into the carbon backbone also enhances the functionality of nanostructured particles [16–20]. One of the methods for the functionalization of carbon materials is the introduction of phosphine derivatives [21–23]. They are important ligands in coordination chemistry, especially in organoelement compounds. Phosphine fragments are often valuable precursors for the production of noncombustible materials, lubricants, extractants, and flotation agents [24].
An alternative method for modifying nanoparticles, consisting in the involvement of complex substrates that contain desired decorating moieties in the synthesis of nanostructures, has not yet received systemic development. In this principle of modification of carbon structures, the heteroatomic component triphenylphosphine is coordinated to a substrate before treatment by a pulsed low-voltage discharge (predischarge-coordinated). Triphenylphosphine is an orbital-controlled (soft) base [25]. During the polycondensation of chloromethanes, PPh3 performs three functions: as an electron-donor component in complexes with substrates, replacing one of the nucleofugic groups in substrates (soft acids), increasing their reactivity; a hydrogen source, providing intermolecular elimination of HCl; and a source of fragments of nanostructures for the functionalization of active particles formed by the action of pulsed low-voltage discharges in liquid media. Thus, the polycondensation induced by low-voltage discharges involves donor–acceptor complexes of substrates with triphen-ylphosphine, rather than individual chloromethanes. Preliminary results on the activating effect of triphenylphosphine on substrates are reported in [26, 27].
This communication presents a map of distribution of elements (scanning electron microscopy–energy dispersive X-ray spectroscopy mapping) in nanostructures and the results of thermal adsorption determination (by direct evolved gas analysis–mass spectrometry) of the composition of the components of solid structures obtained by low-voltage discharge-induced polycondensation of chloromethane complexes with triphenylphosphine. The distribution map of the elements of nanostructures produced by the action of low-voltage pulsed discharges on complex substrates is used to evaluate the concentration of elements (C, P, Cl) in local regions and on the entire surface of nanostructures. The composition of the formed nonvolatile products and components distributed in the solid phase was determined from thermal desorption analysis spectra. In this case, the order of appearance of fragments in the spectrum, taking into account their molecular weight, was used to assess the possibility of interaction of components with the surface of nanostructures (decorated nanostructures) or finding components in the cavity of nanostructures (encapsulated components).
EXPERIMENTAL
The following chemicals were used in the study: dichloromethane (boiling point 39.6°C, 99.9 mol %), trichloromethane (boiling point 61.2°C, 99.9 mol %), tetrachloromethane (boiling point 76.7°C, 99.9 mol %), and triphenylphosphine (boiling point 377°C, 99.0 mol %). In the experiments on polycondensation induced by low-voltage discharges, binary systems of CH2Cl2, CHCl3, and CCl4 with PPh3 (9 wt %) were studied. To illustrate the contribution made by PPh3 to the change in the elemental and component composition of the polycondensation products of chloromethanes, in this paper we present the results of the discharge-induced polycondensation of pure CHCl3.
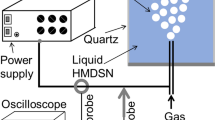
Figure 1 shows a schematic of an experimental setup with a reactor volume of 40 cm3. Graphite electrodes of 6 mm in diameter are installed inside the reactor. A 60-V dc voltage source (VS) with a capacitance of 2200 μF was used to generate low-voltage discharges by varying the interelectrode gap. The discharge duration and power were regulated using a control unit. Under these conditions, the discharge energy is sufficient to break bonds and initiate chemical reactions [28]. The discharge control unit and the energy calculation procedure are detailed in [29].
Schematic of the experimental setup: reactor (R); reflux condenser (RC), electrodes (E), manometer (M), valve (V), gas trap (GT), clutch (C), stepper motor (SM), stepper motor driver (D), stepper motor power supply (PS), current sensor (CS), microprocessor control system (MCS), and voltage source (VS).
A reactant was loaded into the reactor at ambient temperature in a helium atmosphere to prevent oxidation. The liquid level in the reactor was well above the level of electrodes E. To prevent evaporation of the reactant, a reflux condenser RC (temperature 4°C) was used. The temperature in the reactor did not exceed the reactant boiling point. The resulting gas was cooled and trapped in a gas trap (GT). The pressure in the reactor was atmospheric.
To assess the effect of admixture of triphenylphosphine to chloromethane on the conversion by low-voltage discharge treatment, the effects of reactants alone and the reactants with triphenylphosphine admixtures to plasma were studied. Table 1 shows the experimentally measured values of conversion, gas yield, and yields of solid products for the systems under study.
Thermal desorption analysis (Direct EGA-MS) for the composition of solid products was performed on a temperature-programmable Double-Shot EGA/PY-3030D pyrolyzer (Frontier Laboratories, Japan) built into a Shimadzu QP-2010Plus GC–MS system (Japan). For the analysis, 100 μg of the sample was loaded into a stainless steel bowl and placed in a fused-silica pyrolysis tube. The fused-silica tube was surrounded by a tube furnace to provide uniform heating. The first stage of the analysis was holding at 323 K for 10 min, after which the sample was heated in the range of 323–773 K (10 K/min). At the second stage, the chromatographic peaks obtained were identified using a mass selective detector. The mass spectrometer operated in electron ionization (EI) mode at 70 eV with scanning in the mass range from 12 to 500 amu. Reaction products were identified using the NIST-11 mass spectra database and the GCMS Real Time Analysis® software.
To determine the elemental composition of the samples, the surface was examined by energy dispersive X-ray spectroscopy (EDS) using a Jeol JSM-6700F scanning electron microscope (Japan). The samples were fixed to a sample holder with a conductive carbon tape and coated with a 3 nm Au/Pd film by magnetron sputtering (Q150R S, Quorum, the United Kingdom) to compensate for the electron beam-induced charge. The morphological and visual appearance characteristics were visualized using scanning electron microscopy (Merlin, Carl Zeiss, Germany) at an electron acceleration energy of 5–7 kV with a secondary electron detector built into the lens. The vacuum in the microscope chamber was ~10−6 mbar. A conductive gold layer of a 20 nm thickness was deposited onto the samples.
RESULTS AND DISCUSSION
Electron microscopy images of solid structures (Fig. 2) show that in the case of the low-voltage discharge treatment of the CH2Cl2 + PPh3 complex system, there are visible nanostructure regions having areas where the structures are asymmetrically organized in shape (whiskers, lamellas, etc.). These areas differ greatly in elemental composition (Table 2). The area of the structures in the region of spectrum 3 of the image (Fig. 2) has the form of lamellas with phosphorus and carbon contents of 26.3 and 73.7 at %, respectively. In these nanostructures, phosphorus atoms are uniformly distributed over the entire area (Fig. 3). A portion of the map (Fig. 2, spectrum 2) is formed by structures with a low phosphorus content (the concentrations of carbon, chlorine, and phosphorus are 94.8, 0.5, and 4.7 at %, respectively).
The high phosphorus content and the low chlorine content (Fig. 3) in the solid products of the induced polycondensation of the CH2Cl2 + PPh3 system are due to the participation of PPh3 both directly in the formation of a chain of nanostructures (formation of C–P bonds) and in the decoration of the resulting structures with phosphorus-containing fragments.
The results of the CHCl3 conversion are presented to illustrate the changes, caused by due to the presence of triphenylphosphine, in the transformation characteristics of chloromethanes. Comparison of local regions (Table 3, spectra 10–12 and 4–9) of the solid products clearly shows the effect of triphenylphosphine additives on the elemental composition. During the induced polycondensation of pure trichloromethane, structures are produced in the form of a cactus (Fig. 4, spectrum 9) having a diameter of 15–25 µm, a carbon content of 56.8 at %, and a high chlorine content of 43 at %. These spheres are connected by single (400–600 nm in diameter) and woven (up to 7 µm in diameter) threads with carbon and chlorine concentrations of 84.3 and 15.7 at %, respectively (spectrum 6). The nanostructures produced by low-voltage discharge-induced polycondensation of the binary system CHCl3 + PPh3 (Fig. 5) differ significantly from the structures of polycondensation of pure CHCl3 (Fig. 6) by a very low chlorine concentration (0.6–0.9 at %). In shape, local groupings of structures have the form of long tubes (spectrum 10) or fine particles (spectra 11, 12).
The effect of triphenylphosphine is especially pronounced during the polycondensation of CCl4. Without the “predischarge” activation of PPh3, tetrachloromethane is transformed very slowly [26]. In the presence of PPh3 (9 wt %), the conversion of the CCl4 + PPh3 binary system by the discharge treatment reaches 71 wt % in 20 min. The main solid products of condensation of the CCl4 + PPh3 system are structured carbon compounds of whisker forms (Fig. 7). According to the SEM image, the carbon nanostructures occupy the main part of the solid phase.
In the case of the low-voltage discharge-induced polycondensation of the PPh3 + CCl4 system, the PPh3 additive acts as a predischarge activator and a hydrogen source for intermolecular elimination of HCl. During the transformation of the complex system, the formation of molecular chlorine via α-elimination during the fragmentation of tetrachloromethane is apparently hindered because of the ease with which the eliminated chlorine is involved in the continuation of the reaction. In the case of pulsed discharge treatment, such elimination without hydrogen sources becomes actually reversible [30].
Elemental analysis of the solid sample shows that the main part of the grouped structures is formed from carbon alone (Fig. 8 and Table 4, spectra 13 and 15). Only in certain regions (spectrum 14), the structures include chlorine (8.7 at %) and phosphorus (4.4 at %) atoms.
Binary systems are donor–acceptor complexes in which one of the chloromethane chlorine atoms is replaced with triphenylphosphine to form a P–C bond [26]. The formation of a binary system of di-, tri-, or tetrachloromethane with PPh3 under the action of low-voltage pulsed discharge can be represented as a Sn2 process (reaction (1)).

Thus, in binary systems, the PPh3 additive acts as a source of phosphorus-containing decorating fragments. In this case, PPh3 enters into the reaction in the form of a component of complex systems with chloromethanes prior to the low-voltage discharge treatment.
The action of a pulsed low-voltage discharge on complex I in a solution of any of the chloromethanes under study results in the formation of supermolecular carbon structures, such as whiskers, spheres, and lamellas. The atomic composition of the structures in local regions is given in Tables 2–4. Although the complexes of di-, tri-, and tetrachloromethanes with PPh3 are similar in constitution, the structures formed by the discharge treatment differ in morphology and elemental and component compositions. The morphology of the structures changes greatly on passing from one substrate to another (Figs. 2, 4, 7).
The thermal desorption spectrum of nanostructures obtained in pulsed low-voltage discharge-induced polycondensation of dichloromethane predischarge-activated with PPh3 shows the presence of some compounds whose formation is difficult to predict. In this case, components in the solid, liquid, and gaseous states are often encapsulated inside structures, such as nanotubes. As shown in [31–33], molecules undergo self-organization and nanotubes not only retain both simple and complex substances inside them, but also are aligned into chains (peapods), which are structures consisting of fullerenes encapsulated in nanotubes.
The spectrum given in Table 5 shows that there are several routes of dichloromethane transformation by the action of low-voltage discharge. In the formation of trichloromethane, the dichloromethane molecule is involved in two states, triplet and singlet [26]. Chloromethanes are partially transformed into the triplet state by low-voltage discharge treatment according to scheme 1 (reaction (2)). The combination of dichloromethyl and chlorine radicals is a barrierless (reaction (3))


The generated chloromethyl radicals are involved in the “assembly” of nanostructures immediately at the relaxation step or after additional transformation. The second route of dichloromethane conversion (reaction (4)) is participation in the formation of triphenyl(chloromethyl)phosphonium chloride under the action of electric discharge.

Hydrogen chloride, which appears in the spectrum and is retained during desorption to a temperature of 400 K, is apparently bound in the structures into a complex anion with triphenyl(chloromethyl)phosphonium chloride (reaction (5)) [34, 35].

Triphenylphosphine oxide is most likely formed as a result of the oxidation of PPh3 with atmospheric oxygen during drying of the solid phase after the pulse low-voltage discharge treatment. The formation of acetonaphthene most likely results from assembling valence-unsaturated active species generated by low-voltage discharges in a liquid medium.
A comparison of the thermal desorption spectra of the products of low-voltage discharge-induced condensation of CHCl3 (Table 6) and CHCl3 + PPh3 (Table 7) reveals the PPh3 effect, which manifests itself in a change in the component composition of the polycondensation products. Triphenylphosphine dramatically alters the spectrum of thermal desorption of nanostructures produced by the low-voltage discharge-induced polycondensation of trichloromethane. The main components appearing in the spectrum of trichloromethane polycondensation nanostructures are hexachloroethane and hexachlorobenzene. In addition, hexachlorobutadiene and octachloronaphthalene appear in small amounts in the thermal desorption spectrum.
The thermal desorption spectrum of the solid products of polycondensation of the CHCl3 + PPh3 system shows that the main products are triphenylphosphine, triphenylphosphine oxide, and triphenylchloromethylphosphonium chloride. The products also contain hydrogen chloride, which is retained in solid products up to a temperature of 850 K. This anomaly in the behavior of HCl, according to [34], is due to the binding of HCl to the complex anion (reaction (5)). The use of triphenylphosphine as an activa-ting component in the conversion of chloromethanes makes it possible to produce highly demanded chlorinated products that are difficult to obtain by other methods [35].
In such a situation, the order of appearance of components or their fragments can be violated in the thermal desorption analysis of solid structures. Based on the results obtained, it is possible to qualitatively represent the relationship between the fragments in the solid-phase part of the products and the composition of the adsorbed and encapsulated components.
The chemical aspect of the triphenylphosphine effect is especially clearly seen in the analysis of the thermal desorption spectra of nanostructures obtained in the electric discharge-induced polycondensation of the CCl4 + PPh3 system. The phenyl moiety acts as a hydrogen donor for the intermolecular elimination of hydrogen chloride and as a scavenger of chlorine atoms generated from tetrachloromethane. The formation of dichloro-, trichloro-, pentachloro-, and hexachlorobenzenes is a clear illustration of the successive replacement of hydrogen atoms of the aromatic ring by chlorine. Active carbon particles generated by the action of low-voltage discharge form nanostructures decorated with chlorine atoms and partially with triphenylphosphine fragments. During thermal desorption, the decorating fragment of triphenylphosphine, having a high nucleofugity, undergoes fragmentation to give triphenylphosphine. Partly, as already noted, triphenylphosphine is obtained through fragmentation of phosphorus-containing fragments decorating nanostructures. Triphenylphosphine oxide manifested in the thermal desorption spectra of the products of discharge-induced polycondensation of all studied chloromethanes with the PPh3 admixture is due to the oxidation of triphenylphosphine with atmospheric oxygen during drying of the samples.
The thermal desorption spectra of nanostructures (Table 8) obtained by the discharge-induced polycondensation of the CCl4 + PPh3 binary system, along with HCl, revealed another anomaly associated with the behavior of benzene in the solid products. Accord-ing to its physical characteristics, benzene as a low-boiling component should not be present in the solid products after drying the samples during sample preparation. The residual amounts of benzene after drying should appear at a temperature of 350–400 K. In fact, benzene appears in the analysis at a temperature of 997 K. This anomaly is most likely associated with the presence of benzene in an encapsulated state inside nanotubes. Benzene molecules are retained in capsules (like milk in a coconut shell) to a temperature at which the walls or plugs of nanotubes are partially destroyed and benzene diffuses through these defects [36, 37]. Self-organization of benzene molecules inside nanostructures is also possible, which causes changes in the physical properties of benzene. A similar phenomenon, called self-organization of molecules, was recently discovered for water molecules [11–13].
CONCLUSIONS
The results obtained in this work show that polycondensation induced by low-voltage pulsed discharges ensures the formation of nanostructures from chloromethanes. The shape of the resulting structures varies widely depending on the chlorine content in the substrates. The results presented in the paper also show that the addition of triphenylphosphine not only results in the predischarge activation of the substrates, but also leads to fundamental changes in the morphology and elemental and component compositions of solid prod-ucts. This PPh3 effect is especially clearly illustrated by the results of the induced transformation of pure CHCl3 and CCl4 and their binary systems with triphenylphosphine. Pure tetrachloromethane remains almost unconverted during its electrical discharge treatment. The conversion of the CCl4 + PPh3 binary system by treatment in low-voltage discharge for 20 min reaches 71 wt %.
Upon polycondensation induced by low-voltage discharges, trichloromethane forms spherical structures with cactus-shaped needles of 15–25 µm in diameter, connected by single (400–600 nm in dia-meter) and woven (up to 7 µm in diameter) filaments. The binary system CHCl3 + PPh3 yields nanostructures in the form of lamellas under the same conditions. Comparison of the thermal desorption spectra of the solid products of polycondensation of CHCl3 and CHCl3 + PPh3 shows that only perchlorinated hydrocarbons with different backbones (linear, cyclic, aromatic) are found in the former case. During the polycondensation of the binary system CHCl3 + PPh3, the spectra show CHCl3; C6Cl6; triphenyl(chloromethyl)phosphonium chloride; and, additionally, HCl, which is released in the temperature range of 450–850 K. The thermal desorption analysis of the solid polycondensation products of the CH2Cl2 + PPh3 system also reveals the intense evolution of hydrogen chloride in the range of 360–800 K. The delay in the appearance of HCl is caused by binding Cl– to the complex anion \({\text{HCl}}_{2}^{ - }.\) Heating during the analysis of polycondensation products of the CH2-Cl2 + PPh3 and CHCl3 + PPh3 systems results in slowly decomposition of the complex of triphenyl(chloromethyl)phosphonium chloride with the solid anion \({\text{HCl}}_{2}^{ - }\) to form HCl [34].
The thermal desorption spectrum of the solid product of polycondensation of the CCl4 + PPh3 system exhibits an abnormality associated with a high benzene release temperature (997 K). This anomaly is most likely due to the encapsulated state of benzene inside the nanostructures.
REFERENCES
Novoselov, K.S., Geim, A.K., Morozov, S.V., Jiang, D., Zhang, Y., Dubonos, S.V., Grigorieva, I.V., and Firsov, A.A., Science, 2004, vol. 306, p. 666.
Novoselov, K.S., Jiang, D., Schedin, F., Booth, T.J., Khotkevich, V.V., Morozov, S.V., and Geim, A.K., Proc. Natl. Acad. Sci. U.S.A., 2005, vol. 102, p. 10451.
Hersam, M.C., Nat. Nanotechnol., 2008, vol. 3, p. 387.
Nanot, S., Haroz, E.H., Kim, J.H., Hauge, R.H., and Kono, J., Adv. Mater., 2012, vol. 24, p. 4977.
Yu, M.F., Files, B.S., Arepalli, S., and Ruoff, R.S., Phys. Rev. Lett., 2000, vol. 84, p. 5552.
Yu, M.F., Lourie, O., Dyer, M.J., Moloni, K., Kelly, R.F., and Ruoff, R.S., Science, 2000, vol. 287, p. 637.
Smith, B.W., Monthioux, M., and Luzzi, D.E., Nature, 1998, vol. 396, p. 323.
Loi, M.A., Gao, J., Cordella, F., Blondeau, P., Menna, E., Bartova, B., Hebert, C., Lazar, S., Botton, G., Milko, M., and Ambrosch-Draxl, C., Adv. Mater., 2010, vol. 22, p. 1635.
Orellana, W. and Vasquez, S.O., Phys. Rev. B: Condens. Matter, 2006, vol. 74, p. 125419.
Wang, Y., Huang, Y., Yang, B., and Liu, R., Carbon, 2008, vol. 46, p. 276.
Agrawal, K.V., Shimizu, S., Drahushuk, L.W., Kilcoyne, D., and Strano, M.S., Nat. Nanotechnol., 2017, vol. 12, p. 267.
Kurita, T., Okada, S., and Oshiyama, A., Phys. Rev. B: Condens. Matter, 2007, vol. 75, p. 205424.
Agrawal, B.K., Singh, V., Pathak, A., and Srivastava, R., Phys. Rev. B: Condens. Matter, 2007, vol. 75, p. 195420.
Liu, H.Y., Zhang, L.N., Yan, M., and Yu, J.H., J. Mater. Chem., 2017, vol. 5, p. 6437.
Yao, X.P., Li, J., Kong, L., and Wang, Y., Nanotechnology, 2015, vol. 26, p. 1.
Biju, V., Chem. Soc. Rev., 2014, vol. 43, p. 744.
Fan, W., Yung, B., Huang, P., and Chen, X., Chem. Rev., 2017, vol. 17, p. 13566.
Chen, F. and Cai, W., Small, 2014, vol. 10, p. 1887.
Alshehri, R., Ilyas, A.M., and Hasan, A., J. Med. Chem., 2016, vol. 59, p. 8149.
Pooresmaeil, M. and Namazi, H., Colloids Surf., B, 2018, vol. 172, p. 17.
Biagiotti, G., Lange, V., Ligi, C., Caporali, S., Muniz-Miranda, M., Flis, A., Pietrusiewicz, K.M., Ghini, G., Brandi, A., and Cicchi, S., Beilstein J. Nanotechnol., 2017, vol. 8, p. 485.
Muleja, A.A., Mbianda, X.Y., Krause, R.W., and Pillay, K., Carbon, 2012, vol. 50, p. 2741.
Muleja, A.A., Environ. Sci. Pollut. Res., 2008, vol. 25, p. 20032.
Marklund, A., Andersson, B., and Haglund, P., Chemosphere, 2003, vol. 53, p. 1137.
Chemical Reactivity and Reaction Paths, Klopman, G., Ed., New York: Wiley–Interscience, 1974.
Bodrikov, I.V., Titov, E.Y., Subbotin, A.Y., Grinvald, I.I., Titov, D.Y., and Razov, E.N., Plasma Process. Polym., 2020, vol. 17, e1900247.
Bodrikov, I.V., Titov, E.Y., Grinval’d, I.I., Titov, D.Y., Kurskii, Y.A., and Razov, E.N., High Energy Chem., 2020, vol. 54, no. 1, p. 72.
Bodrikov, I.V., Kut’in, A.M., Titov, E.Yu., Titov, D.Yu., Kurskii, Yu.A., and Gazizulin, R.R., Plasma Process. Polym., 2018, vol. 15, e1800094.
Titov, E.Y., Titov, D.Y., Bodrikov, I.V., Kut’in, A.M., Kurskii, Y.A., and Gazizzulin, R.R., High Energy Chem., 2018, vol. 52, no. 6, p. 512.
Kovács, T., Turányi, T., Föglein, K., and Szépvölgyi, J., Plasma Chem. Plasma Process., 2005, vol. 25, p. 109.
Cao, J., Wang, Y., Chai, J., and Shi, J., Comput. Mater. Sci., 2019, vol. 160, p. 403.
Smith, B.W. and Luzzi, D.E., Chem. Phys. Lett., 2000, vol. 321, p. 169.
Shiraishi, M., Takenobu, T., Yamada, A., Ata, M., and Kataura, H., Chem. Phys. Lett., 2002, vol. 358, p. 213.
Nikitin, K., Muller-Bunz, H., Muldoon, J., and Gilheany, D.G., Chem.-Eur. J., 2017, vol. 23, p. 4794.
Vetter, A.C., Nikitin, K., and Gilheany, D.G., Chem. Commun., 2018, vol. 54, p. 5843.
Wang, Z., J. Phys. Chem., 2020, vol. 124, p. 3851.
Heo, S. and Sinnott, S.B., J. Appl. Phys., 2007, vol. 102, p. 064307.
ACKNOWLEDGMENTS
The work was carried out with the equipment of the “Modern Nanotechnologies” Ural Shared-Use Center at the Ural Federal University.
Funding
The study was supported by the Russian Foundation for Basic Research, project no. 18-29-24008.
Part of the work related to thermal analysis was carried out within the framework of the basic part of state assignment, project no. 0728-2020-0008 (topic FSWE-2020-0008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by S. Zatonsky
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bodrikov, I.V., Titov, E.Y., Vorotyntsev, A.V. et al. Synthesis of Decorated Carbon Structures with Encapsulated Components by Low-Voltage Electric Discharge Treatment. High Energy Chem 56, 60–69 (2022). https://doi.org/10.1134/S0018143922010039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143922010039