Abstract
It has been found that the fluorescence quantum yield of 3,5-diphenyl-8-CF3-BODIPY luminophores with aminophenyl substituents in aromatic solvents strongly depends on the position of the aniline amino group with respect to the position of attachment to the core of the BODIPY molecule. It has been suggested and substantiated that the reason for quenching of BODIPY meta-isomers is the specific interaction of luminophore molecules with aromatic molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
It is known that fluorescence of luminophores based on derivatives of 4-boron-3а,4а-diaza-s-indacene (BODIPY) can strongly depend on the polarity of the solvent. This is due to the fact that the processes of emission and absorption of light in these molecules are accompanied by intramolecular charge transfer and, in this case, the dipole moment of the molecule can change greatly.
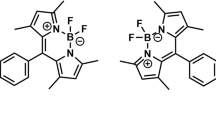
Examples of such compounds are aminophenyl derivatives of BODIPY studied in this work, whose structures are shown in Scheme 1. In these compounds, two fluorine atoms at position 4 and the CF3 group at position 8 are strong electron acceptors, whereas the aminophenyl substituents at positions 3 and 5 exhibit significant electron donating properties. The intramolecular charge transfer for the structures shown in Scheme 1 is most pronounced for compounds P1 and P2, in which the amino group is in the para position with respect to the substitution sites at positions 3 and 5.

Scheme 1 . Derivatives of 8-CF3-BODIPY studied in this work.
In nonpolar solutions of aliphatic hydrocarbons, compounds M1, M2, P1, and P2 have a high fluorescence quantum yield, but in polar solvents, the luminescence efficiency decreases significantly. Intramolecular spatial charge separation arising upon photoexcitation of BODIPY dyes is stabilized by polar aprotic solvents such as 1,4-dioxane, ethyl acetate, acetonitrile, dimethyl sulfoxide (DMSO), and makes these compounds practically nonluminescent [1].
The sharp decrease in the luminescence quantum yield in polar solvents is associated with the fact that, as a result of a strong interaction with dipole solvent molecules, the electronic level of the excited state sharply decreases, which leads to a significant increase in the constant of nonradiative transition to the ground state [2].
At the same time, the luminescence quantum yield of compounds M1 and M2, in which the amino group is in the meta position, decreases in a DMSO solution and in similar polar solvents, but not as significantly as is observed for compounds P1 and P2. This is apparently due to the fact that, due to the weaker interaction of compounds M1 and M2 with dipole solvent molecules, the level of the excited electronic state of compounds M1 and M2 does not decrease as much as it occurs for Р1 and Р2 molecules.
In this work, it has been found that in aromatic solvents (benzene, toluene, and o-xylene), the luminescence characteristics of the luminophores depend very strongly on the position of the aniline amino group with respect to the position of the attachment to the core of the BODIPY molecule. In [3], a special study was carried out on the effects of polarity, polarizability, and pH of solvents on the position of the electronic spectra of molecules with intramolecular charge transfer. To explain the special role of aromatic solvents, a new empirical parameter was introduced, the so-called dipolarity value (SdP), which, along with such values as the polarity and polarizability of the solvent, permits finding an empirical correspondence of the shifts of the absorption and luminescence bands of molecules in which electronic transitions are associated with the internal charge separation. Without going into details, it can be stated that the effect of aromatic solvents is the greater, the more the dipole moment of the luminophore molecule changes as a result of the transition to the excited state. However, in the case of BODIPY molecules, for compounds M1, M2, P1, and P2 in aromatic solvents (benzene, toluene, and o-xylene), the opposite effect is observed: luminescence of meta-isomers (compounds M1 and M2) is quenched much stronger than luminescence of para-isomers (compounds P1 and P2), although the intramolecular charge transfer in the meta-isomers is much less than that in the para-isomers.
Hence, this anomalous behavior of the meta- and para-isomers in aromatic solvents cannot be explained by intramolecular charge transfer. In particular, attempts to use the concept of the dipolarity parameter, according to which benzene and toluene can be conventionally considered as some intermediate case between nonpolar n-hexane and polar solvents, will be fruitless. In that case, quenching of luminescence of the para-isomer should be much greater than that for the meta-isomer, which is not observed in the experiment, whose details will be discussed below. This contradiction served as a motivation for this work.
EXPERIMENTAL
Reagents and Materials
The following solvents were used in the work: n‑hexane (99%, HPLC, Scharlau), cyclohexane (99%, Sigma–Aldrich), benzene (99%, Sigma–Aldrich), toluene (99.99%, HPLC, Fisher Scientific), o-xylene (99%, Sigma–Aldrich). All reagents and organic solvents used were not subjected to additional purification.
Spectral Measurements
The absorption spectra were recorded on a Shimadzu UV 3101PC spectrophotometer and the luminescence spectra on a Shimadzu RF 6000 spectrofluorimeter. The luminescence decay kinetics were measured on a PicoQuant FluoTime 200 time-resolved spectrofluorometer; a semiconductor laser with a wavelength of 467 nm was used as an excitation source.
The luminescence quantum yields were determined at the absorbance of the samples at an excitation wavelength of no more than 0.1. An ethanol solution of rhodamine 6G was used as a standard for measuring the luminescence quantum yield (the quantum yield in an ethanol solution is 95% [4]). When calculating the luminescence quantum yields, the refractive indices of the corresponding solvents were taken into account [5].
All spectral and spectral-kinetic measurements were carried out at room temperature in quartz fluorescent cells with an optical path length of 10 mm. The spectral resolution in luminescence measurements was 3, and 5 nm for samples with a low quantum yield.
1H (400 MHz), 13C (100 MHz), 15N (41 MHz), and 19F (377 MHz) NMR spectra were recorded on a Bruker Avance 400 instrument in CDCl3 and DMSO-d6. Chemical shifts are reported in ppm. The assignment of the signals in the 1H NMR spectra was carried out using COSY and NOESY experiments. The resonance signals of carbon atoms were established on the basis of 1H−13C HSQC and 1H–13C HMBC experiments, and the δ values of 15N on the basis of 2D1H−15N HMBC experiment. Chemical shifts (δ) of 1H are given in relation to the signals of residual protons of deuterated solvents (7.27 ppm for CDCl3 and 2.50 ppm for DMSO-d6), chemical shifts in 13C spectra are given in relation to the peaks of deuterated solvents (77.1 ppm for CDCl3 and 39.5 ppm for DMSO-d6), those in 15N spectra are given in relation to MeNO2 (0.0 ppm), and those in 19F spectra in relation to CFCl3 (0.0 ppm). The spin–spin coupling constants were measured in hertz (Hz) from one-dimensional 1H spectra. IR spectra were obtained on a Bruker IFS-25 spectrometer (400–4000 cm–1, KBr). Microanalysis (C, H, N) was performed on a FlashEA 1112 СHNS-O/MAS instrument (CHN Analyzer). The fluorine content was determined on a SPECOL 11 spectrometer (Carl Zeiss, Jena, Germany). Melting points (without correction) were determined using a StuartScientificSMP3 digital meter.
Quantum chemical calculations for optimization of the geometry of the structures in the ground and excited states were performed using the freely distributed ORCA program [6, 7]. For the ground state, the electronic density functional theory (DFT) was used, and for the excited state, TD–DFT with the PBE0 functional and the 6-311G** basis was used. For the geometry found, the matrix of second derivatives was calculated to make sure that the found minimum is true. The search for the geometry of the excited state began with the optimal geometry found for the ground state. For the optimal geometries found, the energies of vertical transitions were calculated: from the optimized S0 state to S1 without changing the nuclear configuration and from the unoptimized S0 state to the optimized S1 state (also without changing the nuclear configuration).
Molecular dynamics calculations in the NAMD program [8] were used to study the geometric structure of possible complexes of BODIPY with benzene. For this purpose, a cell with periodic boundary conditions was considered, in the center of which a dye molecule was placed, and the rest of the space was filled with benzene. The atomic charges of the dye molecule were taken from the results of quantum chemical calculations [9]. The integration of the classical equations of motion was performed according to the Verlet scheme with velocities with an integration step of 1 fs; the total length of the molecular dynamics trajectory was 1.1 ns, of which the first 0.1 ns was used to minimize the energy. In every 100 steps, the current coordinates of all atoms were saved into a trajectory file, from the analysis of which the possible structures of BODIPY complexes with benzene were found. The structures found were optimized using the ORCA program as described above.
Synthesis of Luminophores M1, M2, P1, and P2
The synthesis of luminophores M1 and P1 is described in detail in [1].
Luminophores M2 and P2 were obtained for the first time by the method described in [10] from 2-(3- and 4-aminophenyl)pyrroles 5a, 5b, which were synthesized from the corresponding acetophenone oximes and acetylene by the Trofimov reaction [11].

Scheme 2 . Scheme of the synthesis of luminophores M2 and P2.
Scheme 2 shows the following stages of the synthesis of luminophores M2 and P2: (i) protection of the amino group of 2-(3- and 4-aminophenyl)pyrroles 1a, 1b by acetic anhydride; (ii) trifluoroacetylation of 2‑(3- and 4-acetamidophenyl)pyrroles 2a, 2b by trifluoroacetic anhydride; (iii) reduction of 2-trifluoroacetylpyrroles 3a, 3b by NaBH4; (iv) condensation of trifluoromethyl(pyrrole)ethanols 4a,b with 2-(3- and 4-acetamidophenyl)pyrroles 2a, 2b; (v) removal of acetamide protection in dipyrromethanes 5a, 5b; and (vi) oxidation/complexation of dipyrromethanes 6a,b into luminophores M2 and P2.
The synthesis and spectral properties of pyrroles 1a,b–4a,b are described in [1].
Synthesis of Dipyrromethanes 5a, 5b
A mixture of pyrroles 2а,b (0.200 g, 1.0 mmol), 4а, 4b (0.298 g, 1.0 mmol), and P2O5 (0.142 g, 1.0 mmol) in MeCN (30 mL) was stirred at room temperature for 24 h. Then NaHCO3 (0.101 g, 1.2 mmol) was added and the reaction mixture was stirred for 1.5 h. The precipitate was filtered off and washed with MeCN (3 × 5 mL). The solvent was removed from the filtrate. Dipyrromethane 5a, 5b was isolated from the precipitate by column chromatography (SiO2, eluent MeCN/n-hexane/CH2Cl2 in a ratio of 20 : 1: 1).
N,N'-[(2,2,2-trifluoroethane-1,1-diyl)bis(1H-pyrrole-5,2-diyl-3,1-phenylene)]diacetamide (5a). Yield 0.226 g (47%), mp 148–150°C.
1Н NMR (400 MHz, DMSO-d6), δ: 11.35 (br s, 2 H, NH), 9.90 (br s, 2 H, NHCO), 7.79 (m, 2 H, H-2 Ph), 7.36 (m, 2 H, H-4 Ph), 7.28 (m, 4 H, H-5,6 Ph), 6.37 (m, 2 H, H-4), 6.22 (m, 2 H, H-3), 5.11 (q, J = 9.5 Hz, 1 H, CH), 2.06 (s, 6 H, Me).
13С NMR (100 MHz, DMSO-d6), δ: 168.2, 139.5, 133.0, 131.5, 129.5, 125.4, 125.4 (q, J = 279.5 Hz, CF3), 118.6, 117.0, 114.7, 109.3, 105.9, 42.1 (q, J = 30.1 Hz, CH), 23.9.
19F NMR (377 MHz, CDCl3), δ: –67.8 d (J = 9.6 Hz).
15N NMR (41 MHz, DMSO-d6), δ: –245.5 (J = 90.8 Hz, NHCO), –226.5 (1JNH = 95.5 Hz, NH).
IR (KBr), ν: 3406, 3303, 3110, 2930, 1672, 1614, 1594, 1540, 1500, 1429, 1384, 1372, 1256, 1189, 1158, 1108, 1075, 1046, 882, 866, 775, 713, 690, 538 cm–1.
Calculated for C26H23F3N4O2 (480.49): C, 64.99; H, 4.83; F, 11.86; N, 11.66. Found: C, 64.72; H, 4.95; F, 11.68; N, 11.58.
N,N'-[(2,2,2-trifluoroethane-1,1-diyl)bis(1H-pyrrole-5,2-diyl-4,1-phenylene)]diacetamide (5b). Yield 0.207 g (43%), mp 154–156°C.
1H NMR (400 MHz, DMSO-d6), δ: 11.23 (br s, 2 H, NH), 9.91 (br s, 2 H, NHCO), 7.55 (m, 8 H, H-2,3,5,6 Ph), 6.39 (m, 2 H, H-4), 6.18 (m, 2 H, H-3), 5.03 (q, J = 9.7 Hz, 1 H, CH), 2.04 (s, 6 H, Me).
13С NMR (100 MHz, DMSO-d6), δ: 168.2, 137.3, 131.5, 127.7, 125.4 (q, J = 279.3 Hz, CF3), 124.9, 123.9, 119.3, 109.3, 105.4, 42.2 (q, J = 30.3 Hz, CH), 24.0.
19F NMR (377 MHz, CDCl3), δ: –67.9 (d, J = 9.7 Hz).
15N NMR (41 MHz, DMSO-d6), δ: –246.5 (J = 90.4 Hz, NHCOMe), –226.9 (J = 95.3 Hz, NH).
IR (KBr), ν: 3402, 3304, 2930, 1673, 1600, 1524, 1426, 1370, 1318, 1254, 1158, 1107, 831, 776 cm–1.
Calculated for C26H23F3N4O2 (480.49): C, 64.99; H, 4.83; F, 11.86; N, 11.66. Found: C, 64.78; H, 5.09; F, 11.63; N, 11.85.
Synthesis of Dipyrromethanes 6a, 6b
A solution of dipyrromethane 5a, 5b (1.201 g, 2.5 mmol) in MeOH (80 mL) was refluxed in the presence of 15% HCl for 2 h. After cooling, water (40 mL) and NH4OH solution (to pH ~ 7–8) were added to the mixture. Then the mixture was extracted with CH2Cl2 (4 × 30 mL), the precipitates were washed with water (3 × 20 mL) and dried over Na2SO4. The solvent was removed and the residue was purified by column chromatography (SiO2, ether) to give dipyrromethane 6a, 6b.
3,3'-[(2,2,2-trifluoroethane-1,1-diyl)bis(1H-pyrrole-5,2-diyl)]dianiline (6a). Yield 0.888 g (90%), mp 126–127°C.
1H NMR (400 MHz, DMSO-d6), δ: 11.19 (br s, 2 H, NH), 7.00 (m, 2 H, H-5 Ph), 6.79 (m, 4 H, H-2,6 Ph), 6.41 (m, 2 H, H-4 Ph), 6.29 (m, 2 H, H-4), 6.16 (m, 2 H, H-3), 5.07 (q, J = 9.8 Hz, 1 H , CH), 5.01 (br s, 4 H, NH2).
13С NMR (100 MHz, DMSO-d6), δ: 148.7, 133.2, 132.3, 129.1, 125.5 (q, J = 279.8 Hz, CF3), 124.8, 112.1, 111.9, 109.4, 109.1, 105.3, 42.0 (q, J = 30.2 Hz, CH).
IR (KBr), ν: 3426, 3396, 2957, 2925, 2864, 1615, 1506, 1471, 1330, 1253, 1194, 1158, 1106, 864, 778, 691 cm–1.
Calculated for C22H19F3N4 (396.42): C, 66.66; H, 4.83; F, 14.38; N, 14.13. Found: C, 66.39; H, 4.99; F, 14.27; N, 14.35.
4,4'-[(2,2,2-trifluoroethane-1,1-diyl)bis(1H-pyrrole-5,2-diyl)]dianiline (6b). Yield 0.829 (84%), mp 182–183°C.
1H NMR (400 MHz, DMSO-d6), δ: 10.93 (br s, 2 H, NH), 7.29 (m, 4 H, H-3,5 Ph), 6.56 (m, 4 H, H-2,6 Ph), 6.15 (m, 2 H, H-4), 6.09 (m, 2 H, H-3), 5.06 (br s, 4 H, NH2), 4.98 (q, J = 9.9 Hz, 1 H , CH).
13С NMR (100 MHz, DMSO-d6), δ: 146.9, 132.6, 125.6 (q, J = 280.3 Hz, CF3), 124.8, 123.7, 121.1, 114.1, 108.8, 103.1, 42.2 (q, J = 30.3 Hz, CH).
IR (KBr), ν: 3430, 2925, 2825, 1622, 1600, 1524, 1479, 1333, 1258, 1158, 1105, 832, 778 cm–1.
Calculated for C22H19F3N4 (396.42): C, 66.66; H, 4.83; F, 14.38; N, 14.13. Found: C, 66.58; H, 4.94; F, 14.25; N, 14.23.
Synthesis of BODIPY M2 and P2
A mixture of dipyrromethane 6a,b (0.595 g, 1.50 mmol) and DDQ (2,3-dichloro-5,6-dicyanobenzoquinone, 0.341 g, 1.50 mmol) in CH2Cl2 (45 mL) was stirred at room temperature for 1.5 h. Then (i‑Pr)2NEt (1.939 g, 15.0 mmol) was added, the resulting solution was stirred for 10 min, and BF3OEt2 (3.193 g, 22.5 mmol) was added dropwise to the solution for 30 min. The reaction mixture was stirred at room temperature for 22 h and diluted with saturated NaHCO3 solution, the organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (3 × 20 mL). The combined organic salts were washed with water (3 × 30 mL) and dried over K2CO3. After removal of solvent from the residue, BODIPY M2 and P2 were isolated by column chromatography (SiO2, eluent n-hexane/ether, gradient from 1 : 1 to 0 : 1 for 2a; and n-hexane/CH2Cl2, gradient from 1 : 1 to 0 : 1 for 4b).
4,4-difluoro-3,5-bis(3-aniline)-8-trifluoromethyl-4-boron-3a,4a-diaza-s-indacene (M2). Yield 0.061 g (9%), mp 164–166°C.
1H NMR (400 MHz, CDCl3), δ: 7.42 (m, 2 H, H-2 Ph), 7.21 (m, 4 H, H-5,6 Ph), 7.17 (m, 2 H, H-1,7), 6.75 (m, 2 H, H-2,6), 6.70 (m, 2 H, H-4 Ph), 3.75 (br s, 4 H, NH2).
13С NMR (100 MHz, СDCl3), δ: 162.2, 146.4, 133.4, 132.9, 130.1, 129.3, 126.5 (q, J = 31.5 Hz, C-8), 123.1, 122.8 (q, J = 276.0 Hz, CF3) , 120.2 (t, J = 2.8 Hz), 117.3, 115.9 (t, J = 4.0 Hz).
IR (KBr), ν: 3442, 3371, 2924, 2859, 1620, 1566, 1509, 1474, 1421, 1306, 1269, 1225, 1135, 1085, 978, 902, 783 cm–1.
Calculated for C22H16BF5N4 (442.20); C, 59.76; H, 3.65; B, 2.44; F, 21.48; N, 12.67. Found: C, 59.84; H, 3.48; F, 21.68; N, 12.79.
4,4-difluoro-3,5-bis(4-aniline)-8-trifluoromethyl-4-boron-3a,4a-diaza-s-indacene (P2). Yield 0.093 g (14%), mp 165–167°C.
1H NMR (400 MHz, СDCl3), δ: 7.81 (m, 4 H, H-2,6 Ph), 7.34 (m, 2 H, H-1.7), 6.71 (m, 2 H, H-2,6), 6.68 (m, 4 H, H-3,5 Ph), 4.00 (br s, 4 H, NH2).
13С NMR (100 MHz, СDCl3), δ: 160.5, 148.9, 133.4, 131.8, 131.7, 128.9, 123.2 (q, J = 276.0 Hz, CF3), 123.0 (q, J = 32.0 Hz, C-8), 122.0, 114.6.
IR (KBr), ν: 3480, 3390, 3216, 2922, 2854, 1623, 1563, 1470, 1446, 1304, 1276, 1226, 1133, 1080, 907, 730 cm–1.
Calculated for C22H16BF5N4 (442.20); C, 59.76; H, 3.65; B, 2.44; F, 21.48; N, 12.67. Found: C, 59.94; H, 3.38; F, 21.25; N, 12.87.
RESULTS AND DISCUSSION
Table 1 summarizes the experimental data on the positions of the absorption and luminescence spectral maxima, Stokes shifts, and luminescence quantum yields and lifetimes. In addition, for comparison, Table 1 shows the same characteristics for 3,5-diphenyl-8-CF3-BODIPY (F2) in different solvents and for P1, M1 in a polar solvent, DMSO, taken from [1].
First, it should be noted that the electron-donating character of aminophenyl substituents is clearly manifested in the bathochromic shift of the absorption and luminescence spectra of the compounds under study, measured in a nonpolar solvent (n-hexane or cyclohexane), as compared to compound F2. It can be seen that the shift for meta-isomers is much smaller (Δλlum = 10 and 16 nm for M1 and M2, respectively) than for para isomers (Δλlum = 38 and 70 nm for P1 and P2, respectively), while the presence of two aminophenyl substituents shifts the luminescence spectrum more strongly than one substituent.
The Stokes shift in nonpolar solvents such as n‑hexane is approximately the same for all these compounds. The same applies to the value of the luminescence quantum yield: in general, it is quite high and is in the range of 80–25%, while a decrease is observed when the luminescence maximum shifts to longer wavelengths. In polar solvents, as already discussed in the introduction, a sharp drop in luminescence (by a factor of 103–104) is observed for all studied aminophenyl derivatives of BODIPY. It is important to note that luminescence quenching for para-isomers is greater than for meta-isomers, while the luminescence quantum yield of F2 remains practically unchanged even in the most polar solvent, DMSO.
Second, attention is drawn to the unusual effect of the aromatic solvents benzene and toluene on the studied aminophenyl derivatives of BODIPY, which was mentioned in the introduction. While for the para-isomers P1 and P2 in benzene and toluene solutions, the quantum yield and lifetime of photoluminescence change insignificantly, for the meta-isomers M1 and M2, the luminescence quenching occurs more than 100 times, but the lifetime does not change. As already mentioned in the introduction, the unusual character of this effect is that the meta-isomers M1 and M2 are characterized by a lower value of intramolecular charge transfer upon absorption of a quantum of light than the para-isomers P1 and P2. This is confirmed by the data in Table 1. Indeed, the Stokes shift and luminescence quenching in a polar solvent, DMSO, are much greater for the para-isomers than for the meta-isomers, while in aromatic solvents (benzene and toluene), the opposite is true. Therefore, the mechanism for luminescence quenching in aromatic solvents should apparently be sought in some specific interaction controlled by the structural correspondence of the solvent and luminophore molecules. For a more detailed study of the mechanism of this process, we measured the luminescence quantum yields of solutions of M1, M2, P1, and P2 in n-hexane as a function of the concentration of aromatic molecules (benzene, toluene, o-xylene) added to the solution.
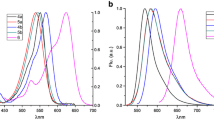
Figure 1 shows for comparison the change in the relative luminescence quantum yield of the meta- (M1) and para- (P1) isomers as a function of the toluene concentration. The addition of only 1 mol % of toluene to the n-hexane solution leads to luminescence quenching of compound M1 by 40%, while P1 is quenched by only 3.5%. It is possible that a specific interaction of toluene occurs for both compounds M1 and P1. However, for compound P1, this interaction is much weaker. The measurement of the same concentration dependences for compounds M1 and P1 in the case of the addition of benzene and o-xylene, as well as for compounds M2 and P2 upon the addition of toluene, benzene, and o-xylene, led to qualitatively similar results. In other words, luminescence of meta-isomers is quenched upon an increase in the concentration of aromatic additives much more strongly than in the case of para-isomers.
Figure 2 presents the results of two series of experiments on luminescence quenching of compounds M1 and M2 in n-hexane solutions as a function of the mole fraction of toluene in the Stern–Volmer coordinates assuming only the dynamic quenching mechanism (Fig. 2a) and mixed quenching (Fig. 2b), that is, dynamic and static quenching simultaneously.
Dynamic luminescence quenching is described by the following equation:
where kq is the bimolecular rate constant of the dynamic quenching reaction of the excited state of the luminophore molecule, τ0 is the luminescence decay time in the absence of a quencher, [Q] is the concentration of a quencher, and KD = kqτ0 is the Stern–Volmer dynamic quenching constant. Static quenching occurs when a quencher molecule forms a nonluminescent complex with a luminophore molecule. In this case, the luminescence quenching is described by the following equation:
where KS is the equilibrium constant of the chemical reaction of the formation of a nonluminescent complex. In both cases, the concentration dependence should be linear in the Stern–Volmer coordinates: φ0/φ → [Q]. As can be seen from Fig. 2a, in our case the luminescence quenching is not described by either a purely dynamic or a purely static quenching mechanism. If quenching occurs by a mixed mechanism, that is, by two mechanisms simultaneously, then the dependence of φ0/φ on the quencher concentration is quadratic [12]:
In this case, the concentration dependence should be linear in the following coordinates:
It can be seen from Fig. 2b that the experimental data are well linearized in coordinates (2), that is, for the mixed quenching mechanism. These experimental data are described by Eq. (1) with the reliability criteria R2 = 0.9994 and 0.9991 for compounds M1 and M2, respectively, evaluated by the least squares method. It is easy to see that the slope of the linear plots in Fig. 2b determines the product of the dynamic and static quenching constants, and the ordinate intercept determines the sum of these constants. For М1, the solution of the corresponding quadratic equation allows determining the values of the constants up to permutation: KD = 5.5, KS = 1.3 [M–1] (KS = 5.5, KD = 1.3 [M–1]). For M2, there are no real solutions for the constants. Hence, the quenching mechanism for luminophore M2 with two aminophenyl groups is more complex.
As a working hypothesis, we assumed that luminescence quenching of the molecule of luminophore M2 with two aminophenyl groups could occur as a result of the formation of two complexes of compositions 1 : 1 and 1 : 2; that is, the luminophore molecule can form a complex with both one and two quencher molecules. Figure 3 shows the structure of such complexes. The complexes are formed as a result of the simultaneous interaction of the benzene ring with the amino group and fluorine atoms.
If we accept this hypothesis about the presence of two complexes, then the dependence of the luminescence quantum yield on the quencher concentration will have the following form:
where KS2 is the equilibrium constant in the reaction between the 1 : 1 complex and the second quencher molecule. The experimental data for М2 were described by Eq. (3) with the reliability criterion R2 = 0.9994. The solution of the corresponding cubic equation gives a unique and unambiguous set of values of the equilibrium constants: KD = 3.6, KS = 1.9, and KS2 = 0.74 [M–1]. The found values of the quenching constants are in good agreement with the values of KD = 5.5 and KS = 1.3 [M–1] for compound М1.
Thus, it seems that the hypothesis of the possibility of the formation of two complexes makes it possible to interpret the obtained experimental data; however, this raises another problem. The thing is that, regardless of the mechanism of static quenching of luminescence, the experimental data on the decrease in the lifetime of the excited state with growing the quencher concentration should be linear in the Stern–Volmer coordinates, but, as will be shown below, this is not observed. Therefore, we turned to a more detailed study of the luminescence decay kinetics as a function of the toluene concentration and the observation wavelength.
As a result of the studies carried out, it was found that for para-isomers P1 and P2, the luminescence decay kinetics has a single-exponential character, and with a decrease in the luminescence quantum yield on passing from n-hexane to an aromatic solvent, a regular decrease in the luminescence lifetime is observed (see Table 1), whereas for meta-isomers M1 and M2, a complex two-exponential kinetics of luminescence decay is observed. Let us consider the data obtained for these compounds in more detail.
Figure 4 shows the effect of addition of benzene, toluene, and o-xylene on the form of the curves of the luminescence decay kinetics of compounds M1 and M2 in n-hexane. It can be seen that in pure n-hexane, the curves for both compounds are almost single-exponential, while additives of toluene significantly complicate their form. This form of the curves obtained in the presence of toluene may be due to the fact that each of the studied compounds M1 and M2 is a mixture of two luminophores, and aromatic molecules (benzene, toluene, and o-xylene) exhibit quenching properties only in relation to one of them. In the case of M1 and M2, the manifestation of this kind of “two-component character” can be associated, for example, with the fact that both compounds are present in solution in the form of at least two different conformers.
Experimental data on the luminescence decay kinetics for compound M1 as a function of the addition of (a) benzene, (b) toluene, (c) o-xylene, and (d) for M2 as a function of the addition of toluene. The upper (red) curve in each figure refers to the luminescence decay kinetics in bulk n-hexane. The excitation wavelength is 470 nm; the kinetics were measured at the luminescence maximum at a wavelength of 632 nm. Solid black curves result from processing of the experimental data following Eq. (4).
Another alternative, which is associated with the possible presence of an uncontrolled impurity of luminescent molecules of a similar structure, will be considered separately at the end of this work.
To simplify further processing of the experimental data, we assume that each of the compounds M1 and M2 is present only as two conformers, one of which interacts with the quencher, and the other does not. In this case, the following equation can be used to process the kinetic curves of luminescence decay:
where PL is the luminescence intensity; t is the time after pulsed excitation; τ1 and τ2 are the luminescence lifetimes for the 1st and 2nd luminescent conformers, respectively; and A1 and A2 are the preexponential factors that determine the relative contributions for the 1st and 2nd conformers, respectively.
The numerical values of the lifetimes and preexponential factors obtained by processing of the kinetic curves of the luminescence decay using Eq. (4) are presented in Table 2. It can be seen that the concentration dependences for compounds M1 and M2 are well described by Eq. (4). However, for compound M1 with one amino group, we obtain with good reliability two lifetimes τ1 and τ2 even at the first additive of toluene, while for compound M2, this is observed starting from a toluene concentration of 0.45 mol/L. This is apparently due to the fact that in compound M2, the second component, which is not quenched by toluene, is much less with respect to the first component (which is quenched) than in the case of compound M1.
In order to obtain additional information about these forms of the luminophore, we measured time-resolved emission spectra (TRES) at a toluene concentration in n-hexane equal to 0.86 mol/L.
The TRES data are obtained by measuring a set of luminescence decay kinetics recorded at different observation wavelengths, which are a function of two variables PL(λ, t), where λ is the wavelength of luminescence detection. In the case of a mixture of two different luminophores, the function PL(λ, t) is represented as
where g1(λ) and g2(λ) are the normalized luminescence spectra of the two assumed forms of the luminophore, and \({{\xi }_{1}}\) and \({{\xi }_{2}}\) are their relative molar fractions.
Figure 5 shows the results of decomposition of the experimental data using Eq. (5). It can be seen that for compounds M1 and M2 at a concentration of toluene in n-hexane of 0.85 mol/L, there are two luminescent forms of the luminophore, whose spectra differ insignificantly, but the lifetimes of the excited states of these forms differ by an order of magnitude.
Luminescence spectra of two forms for compounds M1 (left) and M2 (right) obtained from the decomposition of the TRES matrix into two components following Eq. (5).
We may assume that the two forms of the luminophore are two conformers of the luminophore molecule shown in Fig. 5. The conformers differ from one another by the mutual arrangement of the amino group and the BF2 group of the BODIPY core. The transition from one conformer to another occurs as a result of rotation of the aminophenyl group around the single bond with which it is attached to the BODIPY core at positions 3 or 5. It can be seen from Fig. 6 that a complex of the BODIPY molecule with the aromatic molecule can be formed only for the meta-isomers of conformer A.
If we assume that conformer B is not quenched by an aromatic molecule (that is, does not form a corresponding complex) and conformer A is quenched both statically and dynamically, then the dependence of the luminescence quantum yield on the quencher concentration will be determined by the following equation:
where KB is the equilibrium constant between the conformers. It is not possible to determine the constant KB from the spectral data with acceptable accuracy, since even at KB = 0 a good fit of the experimental data to Eq. (6) is obtained. Instead, we may use the data on the quenching kinetics presented in Fig. 4 and Table 2. From Table 2, the equilibrium constant KB ≈ 0.03 was obtained, a low value of which means that the constants KD and KS almost do not change when the second conformer is introduced into the model.
When adding the second conformer to the model for compound M2 and performing a similar procedure for approximation of the kinetic curves (see Fig. 4 and Table 2), we obtain the value of the constant KB ≈ 0.003. In this case, the introduction of the second conformer into the model also does not lead to a change in the quenching constants KD, KS, and KS2.
Quantum chemical calculations of the electronic structure of the studied compounds and computer simulation of BODIPY molecules and their complexes with benzene were carried out.
To understand what complexes there can be formed, we first used the molecular dynamics method, in which a dye molecule was surrounded by ≈ 103 benzene molecules. The system was observed for 1000 ns, and the most stable motifs of the interaction of the dye with benzene were noted. Then, for the found applicants for the complexes, the geometry was optimized in the quantum chemical program. As a result, several stable structures were obtained for complexes of M1 with benzene, characterized by the location of a benzene molecule in a kind of cavity formed by the BF2 group with the aminophenyl and phenyl rings. The interaction occurs with one or two hydrogen atoms of the phenyl rings with fluorine atoms, as well as all six carbon atoms with the hydrogen atom of the NH2 group. The largest energy gain is 5 kcal/mol for the structure shown in Fig. 6. Complexes with one or two benzene molecules for dye M2 have a similar structure. In this case, the largest energy gain is about 11 kcal/mol, which is quite consistent with the low numerical values of KS and KS2.
For other positions of benzene near М1 and М2 molecules, as well as for Р1 and Р2 molecules, the formation of stable BODIPY–benzene structures could not be found. Therefore, if complexes of M1 and M2 with benzene are formed, then their structure should be described by the structures shown in Fig. 6, and is due to the specific interaction of the quencher with the dye.
It can be seen from Table 3 that, as a result of the specific interaction of the benzene molecule with meta-isomers, the energy of vertical S0 → S1 transitions decreases. In this case, the benzene molecule stabilizes the state with a large dipole moment. Below are the diagrams of the energy levels for the individual dyes and their complexes with benzene for M1 (Fig. 7) and M2 (Fig. 8).
In conclusion, we will return to the discussion of the abovementioned alternative for explanation of the observed experimental data, associated with the possible presence of an uncontrolled impurity of luminescent molecules of a similar structure. Indeed, during the synthesis of compound M1, it is possible that we did not completely remove compound 3-(3-acetamidophenyl)-5-phenyl-8-CF3-BODIPY, whose spectral and luminescent properties were previously described in the article [1]. However, for compound M2, we have no direct evidence of the presence of an uncontrolled luminescent impurity. Nevertheless, it is impossible to vigorously reject this alternative. Therefore, we consider the proposed interpretation of the observed experimental data as a possible hypothesis, since we also do not have direct experimental evidence of the presence of conformers of compounds M1 and M2.
CONCLUSIONS
As a result of the studies performed, we have shown that luminescence of the derivatives of dye BODIPY with the amino group in the meta position, which we considered in this work, is sensitive to the presence of nonpolar aromatic compounds in a mixture with other nonpolar compounds. Noticeable changes in the spectral properties of the dye occur when the mole fraction of benzene or toluene in their mixture with n-hexane is below 1%. The reason for such a sharp change in the spectral properties may be the complexes that are formed between the meta-isomers and the aromatic molecule. The formation energy of such complexes is close to the energy of a conventional hydrogen bond (3 kcal/mol), which makes them quite stable at room temperature.
REFERENCES
Petrushenko, K.B., Petrushenko, I.K., Petrova, O.V., Sobenina, L.N., Ushakov, I.A., and Trofimov, B.A., Asian J. Org. Chem., 2017, vol. 6, p. 852.
Grabowski, Z.R. and Rotkiewicz, K., Chem. Rev., 2003, vol. 103, p. 3899.
Catalan, J., J. Phys. Chem. B, 2009, vol. 113, p. 5951.
Magde, D., Wong, R., and Seybold, P.G., Photochem. Photobiol., 2002, vol. 75, p. 327.
Parker, C.A., Photoluminescence of Solutions with Applications to Photochemistry and Analytical Chemistry, Amsterdam: Elsevier, 1968.
Neese, F., Comput. Mol. Sci., 2012, vol. 2, p. 73.
Neese, F., Comput. Mol. Sci., 2017, vol. 8.
Phillips, J.C., Braun, R., Wang, W., Gumbart, J., Tajkhorshid, E., Villa, E., Chipot, C., Skeel, R.D., Kale, L., and Schulten, K., J. Comput. Chem., 2005, vol. 26. p. 1781.
Nevidimov, A.V, Russ. J. Phys. Chem. A, 2020, vol. 5, p. 951.
Sobenina, L.N., Vasil’tsov, A.M., Petrova, O.V., Petrushenko, K.B., Ushakov, I.A., Clavier, G., Meallet-Renault, R., Mikhaleva, A.I., and Trofimov, B.A., Org. Lett., 2011, vol. 13, p. 2524.
Trofimov, B.A., Mikhaleva, A.I., Schmidt, E.Yu., and Sobenina, L.N., Chemistry of Pyrroles, Boca Raton, FL: CRC, 2014.
Lakowicz, J.R., Principles of Fluorescence Spectroscopy, 3rd ed., Boston: Springer, 2006.
Treibs, A. and Kreuzer, F.-H., Liebigs Ann. Chem., 1968, vol. 718, p. 208.
Monsma, F.J., Barton, A.C., Kang, H.C., Brassard, D.L., Haughland, R.P., and Sibley, D.R., J. Neurochem., 1989, vol. 52, p. 1641.
Golovkova, T.A., Kozlov, D.V., and Neckers, D.C., Org. Chem., 2005, vol. 70, p. 5545.
McCusker, C., Carroll, J.B., and Rotello, V.M., Chem. Commun., 2005, vol. 8, p. 996.
Hattori, S., Ohkubo, K., Urano, Y., Sunahara, H., Nagano, T., Wada, Y., Tkchanko, N.V., Lemmetyinen, H., and Fukuzumi, S., J. Phys. Chem. B, 2005, vol. 109, p. 15368.
Boens, N., Verbelen, B., and Dehaen, W., Eur. J. Org. Chem., 2015, p. 6577.
Funding
This work was carried out under the state assignment no. AAAA-A19-119070790003-7 and supported by the Russian Government (agreement no. 074-02-2018-286) and the Russian Science Foundation (project no. 19-73-10063).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Tatikolov
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gadomska, A.V., Nevidimov, A.V., Tovstun, S.A. et al. Fluorescence Quenching of 3,5-Diphenyl-8-CF3-BODIPY Luminophores Bearing Aminophenyl Substituents by Aromatic Molecules. High Energy Chem 55, 179–192 (2021). https://doi.org/10.1134/S0018143921030024
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143921030024