Abstract—
The post-spinel phases include compounds with stoichiometry \({{A}^{{2 + }}}B_{2}^{{3 + }}{{{\text{O}}}_{4}}\)\(\left( {A_{2}^{{2 + }}{{B}^{{4 + }}}{{{\text{O}}}_{4}}} \right)\) and structures of the calcium ferrite CaFe2O4, calcium titanate CaTi2O4, and marokite CaMn2O4 types. The structures with a centered Cmcm (Bbmm) and primitive Pnma (Pmcn) and Pbcm (Pmab) cells are distinguished in this family of topologically related compounds with a “marokite” channel formed by six octahedra. The sites A and B are occupied by various cations, in particular, Cr, Al, Mg, Fe, Ca, Ti, Fe, Na, and Si, which implies the formation of solid solutions of a wide compositional range. In nature, such high-pressure phases were found in meteorites, as inclusions in diamond crystals, and in rocks from some metamorphic complexes. This review provides a characterization of natural mineralogical finds, the results of an experimental study of post-spinel phases of various compositions and their solid solutions, as well as crystal chemical simulation and assessment of likely compositions and the areas of stability of compounds with a “marokite” channel. The discrepancy between the results of individual studies indicates the necessity to clarify the stability parameters and probable isostructural transitions, and, ultimately, to improve the classification of post-spinel phases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Spinel is a widespread mineral with a stoichiometry of \({{A}^{{2 + }}}B_{2}^{{3 + }}{{{\text{O}}}_{4}}\)\(\left( {A_{2}^{{2 + }}{{B}^{{4 + }}}{{{\text{O}}}_{4}}} \right)\), the study of which is of great importance in the earth sciences, due to the abundance of various geological formations in rocks. In addition, the spinel-type structure is of petrological and geochemical interest due to its ability to accommodate both tri- and divalent cations, such as Fe2+ and Fe3+. However, the stability of phases with the spinel-type structure is limited, since a phase transition and change in structure occur already under transition zone conditions.
Most spinels decompose to oxides at high pressure (Reid and Ringwood, 1969) or undergo structural phase transitions. Various researchers consider compounds with the structures of calcium ferrite CaFe2O4 (Decker and Kasper, 1957), calcium titanate CaTi2O4 (Rogge et al., 1998), and marokite CaMn2O4 (Giesber et al., 2001) as post-spinel phases. The spinel-type structure transforms into a CF-type structure at pressures above 9–12 GPa, followed by a transition to the CT-type structure at pressures above 20 GPa (Akaogi et al., 1999; Chen et al., 2008). In turn, CaMn2O4 transforms into the structural type of marokite at a pressure of ~30 GPa, and CaTi2O4 at 39 GPa.
It should be noted that not all spinel mineral phases have high pressure polymorphs. The rB/rA ratio, where rB and rA are the ionic radii of B and A, respectively, is one of the key criteria for the appearance of the CF-type structure. CF oxides with rB/rA <0.53 were not detected (Müller-Buschbaum, 2003). Structural compounds of the CT type are less studied due to the higher pressures of their synthesis.
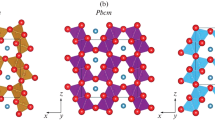
The structure of post-spinel phases is formed by edge and angular octahedra with hollow channels parallel to the b axis (CF-type structure) and a axis (CT-type structure), respectively. These two structures contain eight-fold AO8 and octahedra BO6. There are two types of octahedral BO6 sites in the CF-type structure and one type of octahedral BO6 sites in the CT-type structure (Fig. 1). The structures with a centered Cmcm (Bbmm) and primitive Pnma (Pmcn), Pbcm (Pmab) cells are distinguished within this family of topologically related structures with a “marokite” channel formed by six octahedra.
Unfortunately, at present, post-spinel phases, their properties, and the ability to form the series of solid solutions have not been sufficiently studied. It is necessary to expand the area of the experimental study of these phases and to refine our understanding of them. In this paper, we analyze and systematize the data on the structural patterns and phase transitions of post-spinel phases.
NATURAL POST-SPINEL PHASES
Natural post-spinel phases have been found as inclusions in diamonds, which supports their mantle origin.
A multiphase mineral inclusion in a diamond from the Juina area (Brazil) was described by Kaminsky et al. (2015). It contained iron carbide aggregate (Fe–N–Si–C), Fe-rich periclase (solid solution with magnesioferrite), graphite, as well as Mg–Cr–Fe oxide with the formula (Mg0.90Mn0.18)Σ1.08(Cr1.37V0.11Al0.05)Σ1.92O4 (orthorhombic) and Ca–Cr oxide with the formula (Ca1.07Mg0.02Mn0.02)Σ1.11(Cr1.71V0.06Ti0.03Al0.03)Σ1.89O4 (orthorhombic). In addition to the major elements, there are minor impurities, such as Mg, Al (~0.80 at %), Ti, V, and Fe (~1.93 at %) in Ca–Cr oxide; Al (~1.58 at %) and V, in Mg–Cr–Fe oxide.
Polymineral assemblages were found as inclusions in superdeep diamonds from the Juina-5 kimberlite (Brazil), which were formed during the crystallization of melt with the MORB composition under the lower mantle conditions (Walter et al., 2011). Several high-pressure phases have been described: a phase with a CF-type structure, new hexagonal alumina phase (NAL), Mg-perovskite enriched in Al, Ti, and Fe, and Ti-rich Ca-perovskite.
The composition of the polymineral inclusion in Ju5-20 diamond is similar to the CF phase, while the inclusions in Ju5-67 and Ju5-89 diamonds correspond to the NAL phase formed under the lower-mantle conditions. The synthetic phases are distinguished by significant concentrations of potassium in the NAL phase, while the natural CF phases do not contain potassium (Walter et al., 2011). The natural phase with the calcium ferrite-type structure (CF) contains up to 7.15 wt % Al2O3, 0.04 wt % CaO, and 2.11 wt % FeO. NAL phases contain 7.56 and 6.41 wt % Al2O3, 0.06 and 0.15 wt % CaO, 1.80 and 2.23 wt % FeO, respectively.
In addition to the findings of natural post-spinel phases as inclusions in diamonds, minerals with calcium ferrite- and calcium titanate-type structures were detected in meteorites and impact craters in a number of studies (Table 1).
Thus, maohokite (MgFe2O4), a post-spinel polymorph of magnesioferrite with the CF-type (Pnma) structure, was discovered in impacted gneiss of the Xuyan Crater in China (Chen et al., 2019). This phase coexists with diamond, reidite, TiO2-II, as well as quartz and feldspar diaplect glass and was formed via the subsolidus decomposition of ankerite Ca(Fe2+, Mg)(CO3)2 at a pressure of 25–45 GPa and a temperature of 800–900°C. Xieite (FeCr2O4), a natural orthorhombic chromite polymorph with a calcium ferrite-type structure, was found in shock veins of the Suizhou meteorite (Chen et al., 2008). It was formed via the solid-phase transformation of chromite at high pressures and temperatures, in association with other high-pressure minerals, such as ringwoodite, majorite, etc. According to the phase diagram (Agee et al., 1995), the transformation of chromite into xieite occurs at 18–23 GPa and 1800–1950°C. Chenmingite (FeCr2O4) is a high-pressure mineral with a CF-type (Pnma) structure, which occurs in the form of lamellae in chromite grains in association with xieite and Fe, Cr-saturated ulvöspinel, near veins of melt impactites formed by the impact of the Tissint Martian meteorite. The areas of xieite are always in contact with the melt zones, and chenmingite lamellae are found in chromite only, a few micrometers from these zones. Such position suggests that chenmingite was formed at the same pressure as xieite, but at lower temperatures, which is consistent with the experimental studies (Ma et al., 2019). Tschaunerite (Fe2+)(Fe2+Ti4+)O4 is a high pressure ulvöspinel polymorph. This mineral with the CT-type structure was found in the central part of altered ulvöspinel–ilmenite aggregates in the Tissint Martian shergottite (Ma and Prakapenka, 2018).
As a rule, the natural post-spinel phases are formed at high pressures. Nevertheless, some of them were formed at low pressures (Table 1). Among them are ellinaite (CaCr2O4), which was detected in gehlenite–rankinite paralavas in the southern part of Hatrurim Basin (Negev Desert, Israel) (Sharygin, 2019), marokite (CaMn2O4) found in the dumps of Vein 2 of the Tachgagalt deposit (Gaudefroy et al., 1963), harmunite (CaFe2O4, natural calcium ferrite CaFe2O4 found in larnite-bearing (high-temperature) metamorphic rocks) (Galuskina et al., 2014), wernerkrauseite (CaMn4+O6, one more “low-pressure” mineral (Pnma) found in altered xenoliths of alkaline basalt from the Bellerberg Volcano, Eifel, Germany) (Galuskin et al., 2016).
Finds of post-spinel phases among inclusions in deep-seated diamonds, in particular their detection in intergrowth with other mantle minerals, illustrate their likely formation in diamond-forming chambers and their presence as accessory phases in the Earth’s mantle. A very wide compositional range of them indicates that they may accumulate aluminum, alkalis, and a number of trace elements at high temperatures and pressures corresponding to the transition zone and lower mantle of the Earth (Irifune and Ringwood, 1993; Kesson et al., 1994; Perrillat et al., 2006). In turn, the high concentrations of these elements may be geochemical indicators of the crustal matter in the Earth’s mantle.
EXPERIMENTAL STUDY OF POST-SPINEL PHASES
The conditions of the formation, boundaries of phase transitions (Table 2), and changes in the physical properties of post-spinel phases in various chemical systems were studied experimentally in a wide range of pressures and temperatures. A summary P–T diagram of the stability and phase transitions of post-spinel phases is shown in Fig. 2.
CaFe2O4. Decker and Kasper (1957) reported the synthesis of the compound CaFe2O4 and refined its structure, which turned out to be isomorphous to the CaV2O4-type structure. The study of this phase by single crystal X-ray diffraction showed that, like many other iron oxides, a sharp decrease in its volume occurs at a pressure of ~50 GPa (Merlini et al., 2010). It was shown that a new high-pressure CaFe2O4 phase is formed at a pressure of 51 GPa (Yamanaka et al., 2008). The space group does not change upon the transition, but a shift occurs in every third cation layer. This results in change in the cell parameters: a = 10.0032(11) Å, b = 8.5046(7) Å, c = 2.8366(13) Å at a pressure of 50.2 GPa and a = 9.5834(9) Å, b = 8.2689(13) Å, c = 2.7895(18) Å at a pressure of 52.9 GPa. This was accompanied by a change in the volume of the phase from 241.3(4) to 221.1(6) Å3. The density increased by 8.3% in the new phase. It was shown that this transition was associated with a change of the high-spin state of Fe3+ to the low-spin state (Greenberg et al., 2013).
MgFe2O4. It was shown experimentally that MgFe2O4 with a spinel-type structure begins to transform into a phase with a CaMn2O4-type (CM) structure at a pressure of ~ 17.7 GPa. The diffraction lines of magnesioferrite with a spinel-type structure remain clear up to 27.2 GPa, which indicates mixing of the low- and high-pressure phases between 17.7 and 27.2 GPa. With a subsequent increase in pressure up to 46 GPa, the high-pressure MgFe2O4 phase with the CM-type structure is stable. It was found that the behavior of MgFe2O4 depends on temperature: at T < 1600°C, MgFe2O4 decomposes to form Fe2O3 and MgO, while at T > 1600°C, the MgFe2O4 phase transforms directly into the high-pressure hp-MgFe2O4 phase. Such dual behavior is most likely explained by uneven conditions during diamond-cell experiments. It is believed that magnesioferrite is transformed from the hp-MgFe2O4 phase upon upwelling. However, the phase relations of MgFe2O4 exclude the probability of a direct transition from hp-MgFe2O4 to magnesioferrite. In addition, the stability of hp-MgFe2O4 is not confirmed under the conditions of P > 18 GPa and T = 1000–1500°C or higher. Although the phase is called “magnesioferrite,” compositional analysis shows that it is a solid solution (Mg0.5Fe0.5Fe2O4). At higher pressures, the system contains phases with different stoichiometry: Mg2Fe2O5 and Mg3Fe4O9 (Uenver-Thiele et al., 2017 and references therein). It was shown by Ishii et al. (2020) that the system contains the Mg2Fe2O5 (Cmcm) + Fe2O3 assemblage at pressures of 12–19 GPa, the Mg3Fe4O9 (C2/m) + Fe2O3 assemblage at pressures of 19–22 GPa, while with a further increase in pressure, the MgFe2O4 (Pnma) phase becomes stable. However, despite the same space group Pnma, the structure of the latter phase does not belong to the CF-type structure. The structure of the MgFe2O4 (Pnma) phase has a distorted Z-shaped framework composed of edge-connected (Mg, Fe)O6 octahedra. Octahedra form the channels parallel to the b-axis, which contain two octahedral and one tetrahedral sites. Mg and Fe cations, which do not occupy the framework octahedra, are randomly distributed in the octahedral and tetrahedral sites of the channels, which results in a partial occupation of these three sites. It is assumed that the phase with such structure may be formed in impact craters and meteorites. The authors emphasize the need for careful identification of high-pressure phases by refinement of the crystal structure both in experimental studies and for natural samples, because of the crystallographic similarity of post-spinel structures.
Fe3O4 (Fe2+\({\mathbf{Fe}}_{{\mathbf{2}}}^{{{\mathbf{3 + }}}}\)O4). Magnetite transforms into a high-pressure phase at ~25 GPa. Bassett et al. (1967) suggested that the high-pressure Fe3O4 phase should have monoclinic symmetry. In other studies, the high-pressure phase transition was registered in the range of 25–32.4 GPa, and its temperature dependence was studied. The magnetic properties and electrical resistance changed upon the phase transition as well. It was suggested that the high-pressure Fe3O4 phase crystallized in the CM structure type. Later, the structural type of CaTi2O4 (Bbmm) was suggested for this phase. According to the thermodynamic calculations, Fe3O4 should decompose into FeO and Fe2O3 at 13.3 GPa, and then transform into a high-pressure phase at 35 GPa (Lazor et al., 2004 and references therein).
ZnFe2O4. The ZnFe2O4 phase, which has a mineral name “franklinite,” was studied up to 37 GPa (Levy et al., 2000). A phase with a spinel-type structure is stable up to a pressure of ~25 GPa. The new Zn–Fe phase was detected at 24.4 GPa for the first time. The proportion of a phase with a spinel-type structure in a two-phase aggregate decreases with pressure (with a minimum amount at 36.6 GPa). Based on the results of comparison with other post-spinel structures, the authors suggested that franklinite underwent a transformation from a spinel-like structure to a structure of the CaTi2O4 or CaMn2O4 types.
CoFe2O4. The CoFe2O4 phase was studied up to a pressure of 93.6 GPa (Wang et al., 2003a). The compound has a spinel-type structure up to 32.5 GPa; with further increase in pressure, it transforms to a phase with a CF-type structure, which is stable up to ~94 GPa. The orthorhombic high-pressure phase is 14.7% denser than the tetragonal phase under the normal conditions.
MnFe2O4. The behavior of the jacobsite MnFe2O4 was studied up to a pressure of 39.55 GPa at room temperature (Ye et al., 2015). Spinel with the composition MnFe2O4 undergoes a phase transition at ~18 GPa with the formation of a denser polymorph with the CM-type structure. The high-pressure phase is stable up to 39.55 GPa being preserved after decompression.
MgCr2O4. Based on the data of X-ray diffraction and Raman spectroscopy at high pressure (Yong et al., 2012), it was shown that the symmetry of MgCr2O4 spinel changes from cubic to tetragonal at ~20 GPa and room temperature. According to other data (Ishii et al., 2015 and references therein), spinel of the MgCr2O4 composition transforms into an assemblage of a phase with a modified ludwigite-type structure Mg2Cr2O5 and eskolaite Cr2O3 at pressures of 14–19 GPa and temperatures of 1000–1600°C. With a further increase in pressure and temperature, a transition to a phase with the CT-type structure is recorded (Fig. 2) (Sirotkina et al., 2018), which was first synthesized at 23 GPa and 1600°C and structurally studied by Bindi et al. (2014).
Based on Raman spectroscopy data, the coexistence of two phases was detected in a wide pressure range (14.2–30.1 GPa) (Wang et al., 2002a). The stability of the high-pressure phase was traced up to 76.4 GPa.
FeCr2O4. Chromite FeCr2O4 transforms into Fe2Cr2O5 + Cr2O3 at ~14 GPa and 1200°C, and at higher pressures, into the CF or CT phase (Ishii et al., 2014). The Fe2Cr2O5 phase, like Mg2Al2O5, has the same structure, called the modified ludwigite-type structure (mLd) (Pbam), in which the edge and angular (Fe, Cr)O6 (or (Mg, Al)O6) octahedra are parallel to the c axes, while Fe2+ (or Mg2+) ions occupy the voids in the channels formed by octahedra (Enomoto et al., 2009; Ishii et al., 2014). A sample of natural chromite, similar in composition to the sample from the Suizhou meteorite, was studied at high pressure (Chen et al., 2008). It was shown that FeCr2O4 with a spinel-type structure transforms into the CF phase at 12.5 GPa, and then, into the CT phase at a pressure of >20 GPa.
β-CaCr2O4. There are two CaCr2O4 polymorphs (α and β), which are the high- and low-temperature ones, respectively. Unlike most of chromites with a spinel-type structure, α- and β-CaCr2O4 crystallize with the orthorhombic symmetry, Pmmn and Pnam space groups, respectively. The β-CaCr2O4 phase was studied up to 16.2 GPa. No phase transitions were detected in this range, and the CF-type structure was retained. In addition, it was noted that the structure is more compressible along the a axis than along the b and c axes, which shows an anisotropic elasticity for β‑CaCr2O4 (Zhai et al., 2016 and references therein).
CdCr2O4. The β-CdCr2O4 polymorph crystallizes with a calcium ferrite-type structure (Hill et al., 1956), with rCr/rCd ≈ 0.56. It was shown experimentally that CdCr2O4 spinel was completely transformed into the β-CdCr2O4 phase with the CF-type structure at 10 GPa and 1100°C (Arévalo-López et al., 2010).
ZnCr2O4. Wang et al. (2002b) showed that ZnCr2O4 with the spinel-type structure transforms into the post-spinel phase with the CF- or CT-type structure in the range of 17.5–35 GPa.
NiCr2O4. It was demonstrated experimentally that NiCr2O4 spinel decomposes into oxides Cr2O3 and NiO at 13.1 GPa, the presence of which was traced in situ to a pressure of 57.1 GPa (Wang et al., 2003b).
CaAl2O4. The areas of stability of two phases (low-temperature CA-III and high-temperature CA-IV) coexist in the CaAl2O4 system up to a pressure of 9–10 GPa (Ito et al., 1980; Akaogi et al., 1999). With increasing pressure, both phases transform into the phase with the CF-type structure. This phase was previously obtained by Iskrina et al. (2020) at 15 GPa and 1600°C in association with Ca2Al6O11 compound. Comparison of the CF phase described by Akaogi et al. (1999) and the phase obtained by Iskrina et al. (2020), shows that all structural parameters in the latter work are somewhat lower. This results an underestimated value of the density of the CF-phase (ρ = 3.975 g/cm3) from in the study of Akaogi et al. (1999) in comparison with our value (ρ = 4.08 g/cm3) (Iskrina et al., 2020). Such differences are likely due to the different techniques structure refinement in the considered studies.
MgAl2O4. MgAl2O4 with the CF-type structure was synthesized at 25 GPa and 1600°C (Yutani et al., 1997). A direct transition from MgAl2O4 spinel to the CF phase was previously registered in experiments at pressures of >26 GPa. These parameters are most likely close to the lower limit of stability of MgAl2O4 with the CF-type structure, since in a number of studies, it was shown that with increasing pressure, MgAl2O4 spinel decomposes to form Mg2Al2O5 with the modified ludwigite-type structure (mLd) and Al2O3 at ~20 GPa and 2000°C. (Enomoto et al., 2009; Kojitani et al., 2007 and references therein).
It was shown by Ono et al. (2006) that the MgAl2O4 phase with a spinel structure at a pressure of 30 GPa transforms into a phase with a CF structure. The ε‑MgAl2O4 phase, presumably with the orthorhombic symmetry, previously observed above 25 GPa, was obtained in the range of 40–45 GPa. It was found that ε-MgAl2O4 coexists with periclase and corundum. The diffraction peaks from ε-type MgAl2O4 cannot be identified within the orthorhombic structure. At the moment, the structure of the ε-MgAl2O4 phase and the exact area of its stability have not been determined (Ono et al., 2006 and references therein).
FeAl2O4. Hercynite FeAl2O4 is unstable at pressures of >12 GPa and 1000°C and decomposes to form corundum and wüstite. In later works, the upper limit of hercynite stability was lowered to 8.0 GPa at 1450°C. At 18 and 24 GPa and 1400 and 1600°C, respectively, the decomposition of hercynite occurred as well, but a new high-pressure phase was not detected. Thus, according to the experimental data, the high-pressure FeAl2O4 polymorph with the post-spinel-type structure is unlikely (Schollenbruch et al., 2010 and references therein).
CaMn2O4. The CaMn2O4 compound was synthesized at a pressure of 2.3 GPa and temperature of 600°C, and its structure was refined (Giesber et al., 2001). This phase was studied up to a pressure of 73.7 GPa. At a pressure of ~35 GPa, the transformation of the structure into the CT-type structure begins. In the range of 35–45 GPa, the coexistence of two phases was detected: initial CaMn2O4 and the high-pressure phase with the CT-type structure. With further increase in pressure up to 73.7 GPa, the polymorph with the CT-type structure remains stable. Yamanaka et al. (2008) studied the CaMn2O4 phase up to 75 GPa and specified that the transition to the CT structure occurred at 30 GPa with the decrease in the unit cell volume by 3.8%. In addition, a likely electron-spin transition of Mn3+ in the octahedral site was investigated at this pressure, since the high-spin state may change to a low-spin one. The symmetry of the octahedral MnO6 site increases from “1” in the Pmab space group to “..m” in Bbmm, and then the octahedron becomes more highly symmetric with almost equivalent bonds (Yamanaka et al., 2008 and references therein).
MgMn2O4. The MgMn2O4 compound was studied up to 30 GPa (Malavasi et al., 2005). It was found that this phase had a tetragonal symmetry I41/adm up to 15.6 GPa. There was a phase transition at a higher pressure. The high-pressure compound crystallized with the orthorhombic symmetry and had the CM-type (Pmab) structure. This transition is very similar to the structural change that occurs with the Mn3O4 phase (Paris et al., 1992).
ZnMn2O4. The ZnMn2O4 compound was up to 52 GPa (Asbrink et al., 2006). The phase changes to tetragonal symmetry at 23 GPa and is retained up to 52 GPa. A compound with a post-spinel-type structure is not formed in this range, which differs from the behavior of phases with the different compositions. Spinel ZnMn2O4 with an active Mn3+ Jahn–Teller ion exhibits the first-order structural phase transition at high pressure. The c/a ratio in the tetragonal structure decreases sharply from 1.62 to 1.10 at a transition pressure of ~23 GPa. A high- to low-spin transition was proposed for ZnMn2O4 during the study by X-ray powder diffraction at 23 GPa and by calculation of the band structure; however, experimental results up to 100 GPa do not indicate any change in the electronic state or spin configuration up to 50 GPa (Choi et al., 2006 and references therein).
LiMn2O4. Experiments on the behavior of LiMn2O4 at a pressure of 6 GPa and temperatures up to 1500°C showed a transition to a high-pressure polymorph with the CF-type structure at temperatures above 1100°C (Yamaura et al., 2006).
MgMn2O4. Malavasi et al. (2005) studied the Mg1‒xMn2+xO4 system at 0 ≤ x ≤ 1. It is noteworthy that MgMn2O4 spinel has a tendency to inversion, thus, some of magnesium ions may occur in the octahedral site even at room temperature. When most of the magnesium occupies the tetrahedral site, the tetragonal structure of spinel with the space group I41/adm transforms into the CM-type orthorhombic structure Pmab at a pressure of 15.6 GPa. With increasing amount of magnesium in the octahedra, the boundary of the phase transition from the tetragonal to the orthorhombic symmetry shifts to 14.4 GPa. In addition, it was found that due to an increase in the inversion, the compressibility along the c axis slightly decreases, while the compressibility along the a axis increases.
Mn3O4 (Mn2+\({\mathbf{Mn}}_{{\mathbf{2}}}^{{{\mathbf{3}} + }}\)O4). Paris et al. (1992) studied the Mn3O4 phase up to a pressure of 38.7 GPa. It was shown that a transition from gaussmanite Mn3O4 to the phase with the CM-type structure occurred at 10–12 GPa, which was accompanied by the decrease in volume by 8.7%. However, the unit cell parameters of Mn3O4 (CM) are lower than those of isostructural marokite by 1.6–4.5%. Most likely, this is due to the smaller radius of Mn2+ compared to Ca2+. At 1 atm (10–4 GPa), the Mn3O4 phase with the marokite-type structure is not stable, in contrast to the CaMn2O4 phase. Most likely, this is due to the fact that Mn2+ cation is too small for the site A(VIII), and the structure is destabilized at low pressures.
ZnGa2O4. ZnGa2O4 spinel was studied up to 56 GPa at room temperature (Errandonea et al., 2009). At 31.2 GPa, there is a transition from the cubic spinel-type structure to the tetragonal structure. At 55 GPa, a second transition to the orthorhombic marokite-type (CM) structure occurs. It was shown that ZnGa2O4 is one of the least compressible spinels studied to date.
CaTi2O4. The study of the CaTi2O4 phase by powder X-ray diffraction up to a pressure of 80 GPa showed that a transition to the high-pressure polymorph occurred at 39.6 GPa (Yamanaka et al., 2008). The space group of the high-pressure phase remains the same, while the cell parameters change: a = 9.338(6) Å, b = 9.718(4) Å, c = 3.026(1) Å at a pressure of 29.8 GPa and a = 9.257(5) Å, b = 9.642(3) Å, c = 8.967(2) Å at a pressure of 39.8 GPa. The volume of the phase changes from 274.7(3) to 800.4(2) Å3. The mechanism of the transition is very similar to the above-described transformation registered in CaFe2O4, but, nevertheless, it is simply a martensitic transformation with a rearrangement of atoms in the layer.
Zn2TiO4. The study of the Zn2TiO4 phase at pressures up to 80 GPa showed that Zn2TiO4 spinel initially transforms into the phase of the orthorhombic (CaTi2O4) symmetry at 23.7 GPa, and this phase transition is completed by 32.4 GPa. It was determined that the high-pressure phase of Zn2TiO4 has a higher value of the bulk elastic modulus of 205(6) GPa than that of the cubic spinel phase of 162(11) GPa. In this case, the Zn2TiO4 phase with an orthorhombic symmetry is 2.1% denser than the spinel phase. However, the difference in the density of the Zn2TiO4 (CT) and Zn2TiO4 (Sp) phases is ~10.0% (Zhang et al., 2017 and references therein).
Fe2TiO4. Based on the data of X-ray diffraction, Mössbauer, and Raman spectroscopy, it was shown that ulvöspinel Fe2TiO4 undergoes a series of phase transitions from cubic (Fd3m) to tetragonal (I41/amd) at ~9 GPa, and then to orthorhombic structure (Cmcm) at 12–16 GPa. In this case, the high-pressure Fe2TiO4 phase (Cmcm) is retained upon decompression to normal conditions. In all Fe2TiO4 polymorphs, iron cations are characterized by the high-spin state, which is supported by the data of Mössbauer spectroscopy (Wu et al., 2012). A new Fe2TiO4 high-pressure polymorph was discovered at 48 GPa, but its structure was not refined. With decreasing pressure, this phase transforms back to the Fe2TiO4 polymorph with an orthorhombic symmetry (Cmcm). The orthorhombic Fe2Ti-O4 phase is isostructural to CaTi2O4 (Kyono et al., 2011 and references therein; Wu et al., 2012).
Fe2SiO4. The study of the synthetic phase γ-Fe2SiO4 at pressures up to 66 GPa and at room temperature showed a structural phase transition at ~30 GPa. The high-pressure phase Fe2SiO4 belongs to the space group \(R\bar {3}m\) (Z = 2) (Greenberg et al., 2011). Yamanaka et al. (2015) studied the transition from the cubic spinel structure to the body-centered orthorhombic phase I-Fe2SiO4 (Imma) at ~34 GPa. Laser heating of I-Fe2SiO4 up to 1227°C leads to its decomposition into rhombohedral FeO and stishovite SiO2 (Yamanaka et al., 2015).
CuRh2O4. Along with the tetragonal phase CuRh2O4, Ohgushi et al. (2006) synthesized its orthorhombic analog at a pressure of 4 GPa and a temperature of 900°C. The density of the lower-symmetry phase was higher by 2%.
Co2TiO4. The Co2TiO4 phase with the spinel-type structure is stable up to 21 GPa; with further increase in pressure, it transforms into a high-pressure polymorph with the CM-type structure, and above 35 GPa, a phase with the CT-type structure becomes stable (Zhang et al., 2019).
SOLID SOLUTIONS OF POST-SPINEL PHASES
MgAl2O4–Mg2SiO4 system. The MgAl2O4–Mg2SiO4 solid solution was studied up to a pressure of ~27 GPa and T = 1600°C (Fig. 3a) (Kojitani et al., 2007). This system contains two assemblages in the pressure range up to 23 GPa: Cor + Per with a higher content of the Mg2SiO4 component, and Cor + Per + Grt with a higher content of MgAl2O4 in the starting composition. As the pressure increases, the CF phase appears in the samples, and the CF-free field Cor + Per gradually wedges out with increasing pressure to ~26.5 GPa. The coexistence of garnet and CF at 23 GPa suggests that the phase transition between the assemblages Grt + Per + Cor and Cor + Per + CF or Grt + Per + CF occurs just at this pressure. When the MgAl2O4 content exceeds ~23 mol %, CF becomes the only phase remaining in the system at a pressure of >26.5 GPa.
MgAl2O4–СaAl2O4 system. The MgAl2O4–CaAl2O4 solid solution was studied up to a pressure of ~26 GPa and T = 1200°C (Fig. 3b) (Akaogi et al., 1999). At a pressure of >8 GPa, the CA-IV phase transforms into a phase with the CF-type structure, while the MgAl2O4 phase continues to retain the spinel-type structure (Ito et al., 1980; Akaogi et al., 1999). The MgAl2O4 compound is transformed into a phase with the CF-type structure at 26–27 GPa and is associated with the hexagonal aluminum phase (Hex.P). The CaAl2O4 and MgAl2O4 phases have the different lattice parameters; therefore, despite the absence of immiscibility regions in the system, there is no complete series of solid solutions. However, the solubility of the Mg component in the CF phase increases with pressure. Consequently, magnesium can still enter the structure of calcium aluminates, but under the high-pressure conditions only.
NaAlSiO4–MgAl2O4 system. The NaAlSiO4–MgAl2O4 solid solution was studied up to a pressure of ~30 GPa and T = 1600°C (Fig. 3c) (Ono et al., 2009). At pressures of >17 GPa, the NaAlSi2O6 (Jd) phase decomposes with the formation of the NaAlSiO4 phase with the calcium ferrite-type (CF) structure. This phase is stable up to the maximum pressure studied in this work (30 GPa) with the condition that an admixture of the Mg component does not exceed 30 mol %. With a higher content of magnesium in the structure of NaAlSiO4, the CF phase is accompanied by the hexagonal aluminum phase Hex.P. It should be noted that Na/(Mg + Na) in the CF phase is higher than that in the coexisting Hex.P phase. This is because most of the magnesium, as was noted above, is redistributed into perovskite-like phases, while sodium begins to predominate in the residue, which, accordingly, stimulates the formation of Na-rich phase with the calcium ferrite-type structure in association with Mg- and Na-bearing hp-phase for the starting MORB composition. Guignot and Andrault (2004) confirmed an assumption that the NaAlSiO4 component predominates in the phase with the calcium ferrite-type structure, while the MgAl2O4 component predominates in the hexagonal phase. Based on the fact that sodium prefers to enter the CF phase, it can be assumed that the formation of solid solutions and mixing (probably partial) of the NaAlSiO4 and CaAl2O4 components are likely at high pressures.
Mg2SiO4–MgCr2O4 system. Bindi et al. (2018) synthesized chromium-bearing ringwoodite (4.23 wt % Cr2O3) with an inverse structure at a pressure of 20 GPa and a temperature of 1600°C in the Mg2SiO4–MgCr2O4 system. Inverse ringwoodite is characterized by the incorporation of silicon into the octahedral site and the disordered population of octahedra and tetrahedra with magnesium. Most likely, the inverse structure is stabilized by an impurity of chromium distributed over the octahedral and tetrahedral sites. Earlier, ringwoodite with an inverse spinel-type structure was detected in chromitite from ophiolite of the Luobusa region (Tibet, China) (Griffin et al., 2016). Mg(Mg,Cr,Si)2O4 with a distorted orthorhombic CT-type structure synthesized by Sirotkina et al. (2018) in a wide pressure range (13–18 GPa) in the model system Mg2SiO4–MgCr2O4.
In addition, a solid solution Mg[(Cr,Mg)(Si,Mg)]O4 with a distorted CT-type structure and space group Cmc21, was obtained in the considered system at a pressure of 16 GPa and 1600°C (Bindi et al., 2015).
RESULTS OF COMPUTER SIMULATION OF POST-SPINEL PHASES
Using quantum chemical calculations, it was shown that a phase transition of CaAl2O4 with a change from the P21/m to the Pnma space group occurs at 7–8 GPa (Eremin et al., 2016), which is supported by the experimental data (Lazić et al., 2006). The results of semiempirical simulation by the method of interatomic potentials provided the higher values of the transition boundary (18–19 GPa). In addition, it was shown that the Pnam “marokite” phase is energetically more favorable under the conditions of the Earth’s mantle. Judging by the enthalpy value, the Bbmm phase with a base-centered cell is less preferable under the studied conditions. The same applies to the hypothetical monoclinic phase based on the “α-PbO2” motif, which most likely appears at >130 GPa, but is less favorable than the structures with a “marokite” channel (Marchenko, 2019).
The equations of state of normal spinels MgCr2O4, MnCr2O4, and ZnCr2O4, as well as the reactions of their decomposition into oxides Cr2O3 (eskolaite) and MO (NaCl), were studied using the Hartree–Fock self-consistent field method (Catti et al., 1999). The predicted decomposition pressures for Mg-, Mn-, and Zn-bearing chromium spinels are 19, 23, and 34 GPa, respectively. The spinel-type structure becomes more stable with respect to component oxides as the atomic numbers of metals increase. This effect is especially noticeable for ZnCr2O4. This behavior is likely due to the higher relative stability of the tetrahedral (spinel) and octahedral (NaCl) coordination for Zn2+ than for other M2+ cations, which is consistent with the existence of the tetrahedral phase of wurtzite for ZnO only (Catti et al., 1999).
The MgAl2O4 phase was studied by Catti (2001). According to the results of ab initio modeling (LDA and B3LYP functionals), it was determined that with increasing pressure, the cubic spinel-type structure (Fd3m) transforms into the MgO + Al2O3 assemblage at pressures from 6.4–17.3 to 38.5–57.2 GPa (in each of the ranges, the first number was obtained by the LDA method and the second, by the B3LYP method, respectively), and then it is transformed into a phase with the CT-type structure. According to the experimental data, the range noted above is from 15 to >40 GPa (Irifune et al., 1991; Funamori et al., 1998). It should be noted that Catti (2001) did not consider the MgAl2O4 phase with the CF-type structure.
SIGNIFICANCE OF POST-SPINEL PHASES IN THE EARTH’S MANTLE
High-pressure minerals with the spinel-type structure containing transition elements are important constituents of the lower parts of the Earth’s mantle. Their abundance is a prerequisite for a comprehensive study of phase stability and structure under high pressures and temperatures, especially high-pressure polymorphs. Starting from the transition zone of the Earth, the spinel-type structure ceases to be stable and transforms into a set of simple oxides or into the structures with a “marokite” channel (CM, CF, CT). This is the main reason for both mineralogical and geophysical interest to high-pressure phases with post-spinel types of structures.
The CaFe2O4-type structure is considered as a high-pressure form of many spinels under the conditions corresponding to the transition zone and the upper boundary of the Earth’s lower mantle (Kesson, 1994; Kirby et al., 1996; Funamori et al., 1998). An experimental study of natural basalt samples (Perrillat et al., 2006 and references therein) showed the formation of two Al-rich phases with the CaFe2O4-type structure in association with bridgmanite, Ca-perovskite, and stishovite (Akaogi et al., 1999). The synthesis of similar post-spinel Al phases was carried out before (Gasparik et al., 2000; Litasov and Ohtani, 2005). The incorporation of Si and Na into Al-rich phases increases the stability of the CF-type structure (Irifune and Ringwood, 1993; Kesson, 1994), since NaAlSiO4 has this structure at pressures of >18 GPa (Liu, 1977). The Al phases are the compounds accumulating alkaline elements, such as Na and K. Thus, these post-spinel mineral phases are considered as a likely geochemical reservoir for alkalis and other large cations in the Earth’s mantle.
Along with the most abundant minerals, such as olivine (wadsleyite and ringwoodite) and clinoenstatite (ilmenite), spinel (post-spinel) may contribute to seismic heterogeneities due to stagnation of the subducted lithosphere at the depths of the transition zone (Kirby et al., 1996). This depth range is characterized by a rapid increase in seismic velocities due to the mineral transformations into high-pressure phases in the upper mantle. This explains why the deep seismicity occurs only in the approximate depth range of the transition zone of the mantle, where the minerals of the subsiding slabs should acquire the structures of spinel and ilmenite. Even if the plate penetrates the lower mantle, the seismic heterogeneity should not appear at depths of ~700 km, since the key seismically sensitive phase transformations will be completed already (Kirby et al., 1996). Nevertheless, the study of various characteristics of the post-spinel phases is necessary, especially for understanding of the nature of local seismic heterogeneities in the lower mantle. Ab initio modeling of the elastic properties of the NaAlSiO4, MgAl2O4, and (Mg,Fe)Al2O4 phases with the calcium ferrite-type structure made possible to estimate the velocities and density of the oceanic crust along various mantle geotherms (Wang et al., 2020). It may be assumed that the occurrence of the subducted oceanic crust at significant depths may be the reason for local seismic heterogeneities of the lower mantle due to the ongoing transformations of spinels into post-spinel phases. In addition, the formation of the post-spinel Mg2SiO4-CT phase at high pressure via the interaction of MgSiO3 with the post-perovskite-type (PPv) structure and MgO with the NaCl-type structure was substantiated (Zhang et al., 2017). Due to the relatively small volume of our planet, the further high-pressure phase transition of the MgSiO3(PPv) + MgO(NaCl) assemblage may not seem so important in the study of the Earth’s mantle. However, this may be of decisive importance for understanding of the internal dynamic processes in some exoplanets with masses of ten times higher than that of the Earth.
CONCLUSIONS
Post-spinel phases are a large group of more than 30 mineral phases, mainly with the stoichiometry A2+\({\text{B}}_{2}^{{3 + }}\)O4. Most of them are synthetic, and their natural analogs have not yet been found. Nevertheless, in recent years, six new minerals with a “marokite” channel have been found in nature (harmunite, wernerkrauseite, tschaunerite, maohokite, chenmingite, and ellinaite). The stability fields of natural post-spinel phases are mainly located in high-pressure areas. The discovery of natural phases with the post-spinel-type structure in meteorites and inclusions in diamonds supports the assumption that these compounds are part of the phase composition of the mantle of the Earth and other planets of the solar system. Thus, the study of the formation conditions, likely systems of solid solutions of compounds with a “marokite” channel will allow us to understand the process of the evolution of the composition of matter of the terrestrial planets and, in particular, to assess the effect of subduction of the basaltic oceanic crust to depth on the heterogeneity of the transition zone and the lower mantle.
Change history
06 June 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S001670292218001X
REFERENCES
C. B. Agee, J. Li, M. C. Shannon, and S. Circone, “Pressure-temperature phase diagram for the Allende meteorite,” J. Geophys. Res. 100, 17725–17740 (1995).
M. Akaogi, Y. Hamada, T. Suzuki, M. Kobayashi, and M. Okada, “High pressure transitions in the system MgAl2O4–CaAl2O4: A new hexagonal aluminous phase with implication for the lower mantle,” Phys. Earth Planet. Inter. 115, 67–77 (1999).
D. Andrault and N. B. Casanova, “High-pressure phase transitions in the MgFe2O4 and Fe2O3–MgSiO3 systems,” Phys. Chem. Miner. 28, 211–217 (2001).
Á. M. Arévalo-López, A. J. Dos Santos-García, E. Castillo-Martínez, A. Durán, and M. Á. Alario-Franco, “Spinel to CaFe2O4 transformation: Mechanism and properties of β-CdCr2O4,” Inorg. Chem. 49, 2827–2833 (2010).
S. Asbrink, A. Waśkowska, L. Gerward, J. S. Olsen, E. Talik, “High-pressure phase transition and properties of spinel ZnMn2O4,” Phys. Rev. 60, 12651 (1999).
W. A. Bassett, Takahashi T., Stook P.W. “X-ray diffraction and optical observations on crystalline solids up to 300 kbar,” Rev. Sci. Instrum. 38, 37–42 (1967).
L. Bindi, E. Sirotkina, A. V. Bobrov, and T. Irifune, “X-ray single-crystal structural characterization of MgCr2O4, a post-spinel phase synthesized at 23 GPa and 1600 C,” J. Phys. Chem. Solids. 75, 638–641 (2014).
L. Bindi, E. A. Sirotkina, A. V. Bobrov, and T. Irifune, “Structural and chemical characterization of Mg[(Cr,Mg)(Si,Mg)]O4, a new post-spinel phase with sixfold-coordinated silicon,” Am. Mineral. 100, 1633–1636 (2015).
L. Bindi, W. L. Griffin, W. R. Panero, E. Sirotkina, A. Bobrov, and T. Irifune, “Synthesis of inverse ringwoodite sheds light on the subduction history of Tibetan ophiolites,” Sci. Rep. 8, 5457 (2018).
M. Catti, “High-pressure stability, structure and compressibility of Cmcm-MgAl2O4: An ab initio study,” Phys. Chem. Miner. 28, 729-736 (2001).
M. Catti, F. Freyria Fava, C. Zicovich, and R. Dovesi, “High-pressure decomposition of MCr2O4 spinels (M = Mg, Mn, Zn) by ab initio methods,” Phys. Chem. Miner. 26, 389–395 (1999).
M. Chen, J. F. Shu, and H. K. Mao, “Xieite, a new mineral of high-pressure FeCr2O4 polymorph,” Chinese Sci. Bull. 53, 3341–3345 (2008).
M. Chen, J. Shu, X. Xie, and D. Tan, “Maohokite, a post-spinel polymorph of MgFe2O4 in shocked gneiss from the Xiuyan crater in China,” Meteorit. Planet. Sci. 54, 495–502 (2019).
H. C. Choi, J. H. Shim, and B. I. Min, “Electronic structures and magnetic properties of spinel ZnMn2O4 under high pressure,” Phys. Rev. B – Condens. Matter Mater. Phys. 74, 4–7 (2006).
B. F. Decker and J. S. Kasper, “The structure of calcium ferrite,” Acta Crystallogr. 10, 332–337 (1957).
A. Enomoto, H. Kojitani, M. Akaogi, H. Miura, and H. Yusa, “High-pressure transitions in MgAl2O4 and a new high-pressure phase of Mg2Al2O5,” J. Solid State Chem. 182, 389–395 (2009).
N. N. Eremin, A. E. Grechanovsky, and E. I. Marchenko, “Atomistic and Ab initio modeling of CaAl2O4 high-pressure polymorphs under Earth’s mantle conditions,” Crystallogr. Reports. 61, 432–442 (2016).
D. Errandonea, R. S. Kumar, F. J. Manjón, V. V. Ursaki, and E. V. Rusu, “Post-spinel transformations and equation of state in ZnGa2O4: determination at high pressure by in situ x-ray diffraction,” Phys. Rev. B – Condens. Matter Mater. Phys. 79, 1–20 (2009).
N. Funamori, R. Jeanloz, J. H. Nguyen, A. Kavner, W. A. Caldwell, K. Fujino, N. Miyajima, T. Shinmei, and N. Tomioka, “High-pressure transformations in MgAl2O4,” J. Geophys. Res. Solid Earth. 103, 20813–20818 (1998).
E. V. Galuskin, B. Krüger, H. Krüger, G. Blass, R. Widmer, and I. O. Galuskina, “Wernerkrauseite, \({\text{CaFe}}_{2}^{{3 + }}{\text{M}}{{{\text{n}}}^{{{\text{4 + }}}}}{{{\text{O}}}_{{\text{6}}}}{\text{:}}\) the first nonstoichiometric post-spinel mineral, from Bellerberg volcano, Eifel, Germany,” Eur. J. Mineral. 28, 485–493 (2016).
I. O. Galuskina, Y. Vapnik, B. Lazic, T. Armbruster, M. Murashko, and E. V. Galuskin “Harmunite CaFe2O4: A new mineral from the Jabel Harmun, West Bank, Palestinian Autonomy, Israel,” Am. Mineral. 99, 965–975 (2014).
T. Gasparik, A. Tripathi, and J. B. Parise, “Structure of a new Al-rich phase, [K, Na]0.9[Mg, Fe]2[Mg, Fe, Al, Si]6O12, synthesized at 24 GPa,” Am. Mineral. 85, 613–618 (2000).
C. Gaudefroy, G. Jouravsky, and F. Permingeat, “La marokite, CaMn2O4, une nouvelle espèce minérale,” Bull. Soc. Franç. Minéral. Cristallogr. 86, 359–367 (1963).
H. G. Giesber, W. T. Pennington, and J. W. Kolis, “Redetermination of CaMn2O4,” Acta Crystallogr. Sect. C Cryst. Struct. Commun. 57, 329–330 (2001).
E. Greenberg, L. Dubrovinsky, and C. Mccammon, “Pressure-induced structural phase transition of the iron end-member of ringwoodite γ-Fe2SiO4 investigated by X-ray diffraction and Mössbauer spectroscopy,” Am. Mineral. 96, 833–840 (2011).
E. Greenberg, G. K. Rozenberg, W. Xu, M. P. Pasternak, C. McCammon, K. Glazyrin, and L. S. Dubrovinsky, “Mott transition in CaFe2O4 at around 50 GPa,” Phys. Rev. B – Condens. Matter Mater. Phys. 88, 2–6 (2013).
W. L. Griffin, et al., “Mantle recycling: transition-zone metamorphism of Tibetan ophiolitic peridotites and its tectonic implications,” J. Petrol. 57, 655–684 (2016).
N. Guignot and D. Andrault, “Equations of state of Na–K–Al host phases and implications for MORB density in the lower mantle,” Phys. Earth Planet. Inter. 143, 107–128 (2004).
T. Irifune and A. E. Ringwood, “Phase transformations in subducted oceanic crust and buoyancy relationships at depths of 600–800 km in the mantle,” Earth Planet. Sci. Lett. 117, 101–110 (1993).
T. Ishii, H. Kojitani, S. Tsukamoto, K. Fujino, D. Mori, Y. Inaguma, N. Tsujino, T. Yoshino, D. Yamazaki, Y. Higo, K. Funakoshi, and M. Akaogi “High-pressure phase transitions in FeCr2O4 and structure analysis of new post-spinel FeCr2O4 and Fe2Cr2O5 phases with meteoritical and petrological implications,” Am. Mineral. 99, 1788–1797 (2014).
T. Ishii, H. Kojitani, K. Fujino, H. Yusa, D. Mori, Y. Inaguma, Y. Matsushita, K. Yamaura, and M. Akaogi, “High-pressure high-temperature transitions in MgC-r2O4 and crystal structures of new Mg2Cr2O5 and post-spinel MgCr-2O4 phases with implications for ultrahigh-pressure chromitites in ophiolites,” Am. Mineral. 100, 59–65 (2015).
T. Ishii, N. Miyajima, R. Sinmyo, H. Kojitani, D. Mori, Y. Inaguma, and M. Akaogi, “Discovery of new-structured post-spinel MgFe2O4: Crystal structure and high-pressure phase relations,” Geophys. Res. Lett. 47 (6), e2020GL087490 (2020).
A. V. Iskrina, A. V. Spivak, A. V. Bobrov, N. N. Eremin, E. I. Marchenko, and L. S. Dubrovinsky, “Synthesis and crystal structures of new high-pressure phases CaAl2O4 and Ca2Al6O11,” Lithos. 374–375, 105689 (2020).
S. Ito, K. Suzuki, M. Inagaki, and S. Naka “High-pressure modifications of CaAl2O4 and CaGa2O4,” Mater. Res. Bull. 15, 925–932 (1980).
F. V. Kaminsky, R. Wirth, and A. Schreiber, “A microinclusion of lower-mantle rock and other minerals and nitrogen lower-mantle inclusions in a diamond,” Can. Mineral. 53, 83–104 (2015).
S. E. Kesson, “Phase Relations for the former basaltic crust of the slab in the perovskitite facies of the lower mantle,” Mineral. Mag. 58A, 475–476 (1994).
S. E. Kesson, J. D. F. Gerald, and J. M. G. Shelley, “Mineral chemistry and density of subducted basaltic crust at lower-mantle pressures,” Nature 372, 767–769 (1994).
S. H. Kirby, S. Stein, E. A.Okal, and D. C. Rubie, “Metastable mantle phase transformations and deep earthquakes in subducing oceanic lithosphere,” Rev. Geophys. 34, 261–306 (1996).
H. Kojitani, R. Hisatomi, and M. Akaogi, “High-pressure phase relations and crystal chemistry of calcium ferrite-type solid solutions in the system MgAl2O4–Mg2SiO4,” Am. Mineral. 92, 1112–1118 (2007).
A. Kyono, M. Ahart, T. Yamanaka, S. Gramsch, H. K. Mao, and R. J. Hemley, “High-pressure Raman spectroscopic studies of ulvöspinel Fe2TiO4,” Am. Mineral. 96, 1193–1198 (2011).
B. Lazić, V. Kahlenberg, J. Konzett, and R. Kaindl, “On the polymorphism of CaAl2O4-structural investigations of two high pressure modifications,” Solid State Sci. 8, 589–597 (2006).
P. Lazor, O. N. Shebanova, and H. Annersten, “High-pressure study of stability of magnetite by thermodynamic analysis and synchrotron X-ray diffraction,” J. Geophys. Res. 109, B05201 (2004).
D. Levy, A. Pavese, and M. Hanfland, “Phase transition of synthetic zinc ferrite spinel (ZnFe2O4) at high pressure, from synchrotron X-ray powder diffraction,” Phys. Chem. Miner. 27, 638–644 (2000).
K. D. Litasov and E. Ohtani, “Phase relations in hydrous MORB at 18–28 GPa: Implications for heterogeneity of the lower mantle,” Phys. Earth Planet. Inter. 150, 239–263 (2005).
L. G. Liu, “The system enstatite-pyrope at high pressures and temperatures and the mineralogy of the Earth’s mantle,” Earth Planet. Sci. Lett. 36, 237–245 (1977).
C. Ma and V. Prakapenka, “Tschaunerite, IMA 2017-032a. CNMNC Newsletter, No. 46, December 2018, page 1188,” Eur. Mineral. 30, 1181–1189 (2018).
C. Ma, O. Tschauner, J. R. Beckett, Y. Liu, E. Greenberg, and V. B. Prakapenka, “Chenmingite, FeCr2O4 in the CaFe2O4-type structure, a shock-induced, high-pressure mineral in the Tissint martian meteorite,” Am. Mineral. 104 (10), 1521–1525 (2019).
L. Malavasi, C. Tealdi, M. Amboage, M. C. Mozzati, and G. Flor, “High pressure X-ray diffraction study of MgMn2O4 tetragonal spinel,” Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 238, 171–174 (2005).
E. I. Marchenko, N. N. Eremin, A. Yu. Bychkov and A. E. Grechanovskii, “Ca- and Mg-perovskite phases in the Earth’s mantle as a probable reservoir of Al: computer-simulation evidence,” Moscow Univ. Geol. Bull. 72 (5), 299–304 (2017).
E. I. Marchenko, Extended Abstract of Candidate’s Dissertation in Chemistry (Moscow, MSU, 2019).
M. Merlini, M. Hanfland, M. Gemmi, S. Huotari, L. Simonelli, and P. Strobel, “Fe3+ spin transition in CaFe2O4 at high pressure,” Am. Mineral. 95, 200–203 (2010).
H. Müller-Buschbaum, “The crystal chemistry of AM2O4 oxometallates,” J. Alloys Compd. 349, 49–104 (2003).
K. Ohgushi, H. Gotou, T. Yagi, and Y. Ueda, “High-pressure synthesis and magnetic properties of orthorhombic CuRh2O4,” J. Phys. Soc. Japan. 75, 4–6 (2006).
A. Ono, M. Akaogi, H. Kojitani, K. Yamashita, and M. Kobayashi, “High-pressure phase relations and thermodynamic properties of hexagonal aluminous phase and calcium-ferrite phase in the systems NaAlSiO4–MgAl2O4 and CaAl2O4–MgAl2O4,” Phys. Earth Planet. Inter. 174, 39–49 (2009).
S. Ono, T. Kikegawa, and Y. Ohishi, “The stability and compressibility of MgAl2O4 high-pressure polymorphs,” Phys. Chem. Miner. 33, 200–206 (2006).
E. Paris, C. R. Ross II, and H. Olijnyk, “Mn3O4 at high pressure: a diamond-anvil cell study and a structural modelling,” Eur. J. Mineral. 4, 87–94 (1992).
J. Perrillat, I. Daniel, G. Fiquet, M. Mezouar, and N. Guignot, “Phase transformations of subducted basaltic crust in the upmost lower mantle,” Phys. Earth Planet. Inter. 157, 139–149 (2006).
A. F. Reid and A. E. Ringwood, “Newly observed high pressure transformations in Mn3O4, CaAl2O4, and ZrSiO4,” Earth Planet. Sci. Lett. 6, 205–208 (1969).
M. P. Rogge, J. H. Caldwell, D. R. Ingram, C. E. Green, M. J. Geselbracht, and T. Siegrist, “A new synthetic route to pseudo-brookite-type CaTi2O4,” J. Solid State Chem. 141, 338–342 (1998).
K. Schollenbruch, A. B. Woodland, and D. J. Frost, “The stability of hercynite at high pressures and temperatures,” Phys. Chem. Miner. 37, 137–143 (2010).
V. V. Sharygin, “Orthorhombic CaCr2O4 in phosphide-bearing gehlenite–rankinite paralava from Hatrurim basin, Israel: preliminary data,” In International Conference Magmatism of the Earth and Related Strategic Metal Deposits, Apatity, Russia (Apatity, 2019), pp. 272–276 (2019).
E. A. Sirotkina, A. V. Bobrov, Bindi L., Irifune T. “Chromium-bearing phases in the Earth’s mantle: Evidence from experiments in the Mg2SiO4–MgCr2O4 system at 10–24 GPa and 1600°C,” Am. Mineral. 103, 151–160 (2018).
L. Uenver-Thiele, A. B. Woodland, T. Boffa Ballaran, N. Miyajima, and D. J. Frost “Phase relations of Fe–Mg spinels including new high-pressure post-spinel phases and implications for natural samples,” Am. Mineral. 102, 2054–2064 (2017).
M. J. Walter, S. C. Kohn, D. Araujo, G. P. Bulanova, C. B. Smith, E. Gaillou, J. Wang, A. Steele, and S. B. Shirey, “Deep mantle cycling of oceanic crust: Evidence from diamonds and their mineral inclusions,” Science, 334, 54–57 (2011).
W. Wang, Y. Xu, D. Sun, S. Ni, R. Wentzcovitch, and Z. Wu, “Velocity and density characteristics of subducted oceanic crust and the origin of lower-mantle heterogeneities,” Nat. Commun., 1–8. (2020)https://doi.org/10.1038/s41467-019-13720-2
Z. Wang, H. S. C. O’Neillb, P. Lazorc, and S. K. Saxena, “High pressure Raman spectroscopic study of spinel MgCr2O4,” J. Phys. Chem. Solids. 63, 2057–2061 (2002a).
Z. Wang, P. Lazor, S. K. Saxena, and G. Artioli, “High-pressure raman spectroscopic study of spinel (ZnCr2O4),” J. Solid St. Chem. 165, 165–170 (2002b).
Z. Wang, T. Downs, V. Pischedda, R. Shetty, K. Saxena, S. Zha, S. Zhao, D. Schiferl, and A. Waskowska, “High-pressure X-ray diffraction and Raman spectroscopic studies of the tetragonal spinel CoFe2O4,” Phys. Rev. B – Condens. Matter Mater. Phys. 68, 2–7 (2003a).
Z. Wang, S. K. Saxena, P. Lazor, and H. S. C. O’Neill, “An in situ Raman spectroscopic study of pressure induced dissociation of spinel NiCr2O4,” J. Phys. Chem. Solids. 64, 425–431 (2003b).
Y. Wu, X. Wu, and S. Qin, “Pressure-induced phase transition of Fe2TiO4: X-ray diffraction and Mössbauer spectroscopy. J. Solid St. Chem. 185, 72–75 (2012).
T. Yamanaka, A. Uchida, and Y. Nakamoto, “Structural transition of post-spinel phases CaMn2O4, CaFe2O4, and CaTi2O4 under high pressures up to 80 GPa,” Am. Mineral. 93, 1874–1881 (2008).
T. Yamanaka, A. Kyono, Y. Nakamoto, S. Kharlamova, V. V. Struzhkin, S. A. Gramsch, H. K. Mao, and R. J. Hemley, “New structure of high-pressure body-centered orthorhombic Fe2SiO4,” Am. Mineral. 100, 1736–1743 (2015).
K. Yamato, Q. Huang, L. Zhang, K. Takada, Y. Baba, T. Nagai, Y. Matsui, K. Kosuda, and E. Takayama–Muromachi, “Spinel-to-CaFe2O4-type structural transformation in LiMn2O4 under high pressure,” J. Am. Chem. Soc. 128, 9448–9456 (2006).
L. Ye, S. Zhai, X. Wu, C. Xu, K. Yang, Y. Higo, “Compressibilities of MnFe2O4 polymorphs,” Phys. Chem. Miner. 42, 569–577 (2015).
W. Yong, S. Botis, S. R. Shieh, W. Shi, and A. C. Withers, “Pressure- induced phase transition study of magnesiochromite (MgCr2O4) by Raman spectroscopy and X-ray diffraction,” Phys Earth Planet. Inter., 196-197, 75–82 (2012).
M. Yutani, T. Yagi, H. Yusa, and T. Irifune, “Compressibility of calcium ferrite-type MgAl2O4,” Phys. Chem. Miner. 24, 340–344 (1997).
S. Zhai, Y. Yin, S. R. Shieh, S. Shan, W. Xue, C. P. Wang, K. Yang, and Y. Higo, “High-pressure X-ray diffraction and Raman spectroscopy of CaFe2O4-type β-CaCr2O4,” Phys. Chem. Miner. 43, 307–314 (2016).
Y. Zhang, X. Liu, S. R. Shieh, X. Bao, T. Xie, F. Wang, Z. Zhang, C. Prescher, and V. B. Prakapenka “Spinel and post-spinel phase assemblages in Zn2TiO4: an experimental and theoretical study,” Phys. Chem. Miner. 44, 109–123 (2017).
Y. Zhang, X. Liu, S. R. Shieh, Z. Zhang, X. Bao, T. Xie, F. Wang, C. Prescher, and V. B. Prakapenka, “Equations of state of Co2TiO4-Sp, Co2TiO4-CM, and Co2Ti-O4-CT, and their phase transitions: an experimental and theoretical study,” Phys. Chem. Miner. 46, 571–582 (2019).
ACKNOWLEDGMENTS
The authors are grateful to L.S. Dubrovinsky, professor of the Bavarian Geoinstitute (Bayreuth, Germany), and N.A. Dubrovinskaya, Professor of the Department of Physics of Materials and Technologies under Extreme Conditions, University of Bayreuth (Bayreuth, Germany), for research assistance and valuable scientific advice. The authors thank N.N. Kuz’min (Institute of Spectroscopy, Russian Academy of Sciences) for advice and comments on the crystallographic description of the structures. We would like to thank all researchers working in the field of the subject of this paper but not mentioned in the text due to the space constraints.
Funding
This study was supported by the Russian Science Foundation (project no. 21-17-00147, review of solid solutions of post-spinel phases, discussion of the role of phase transformations in the Earth’s mantle) and by the Russian Foundation for Basic Research (project no. 20-35-90095, review of the composition and structural patterns of post-spinel phases). The study was performed as in accordance with the scientific plan of the Laboratory of Deep Geospheres, Moscow State University, and partly within the framework of the State Task AAAA-A18-118020590140-7 of the Institute of Experimental Mineralogy, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Bobrov
The original online version of this article was revised: Due to a retrospective Open Access order.
Abbreviations. α, α-NaAlO2 phase; Brd, bridgmanite; B3LYP, a type of exchange functional; Ca-Pv, CaSiO3 with the perovskite-type structure; Cor, corundum; CF calcium ferrite-type structure; CM, marokite-type structure; CT, calcium titanate-type structure; Esk, eskolaite; Grt, garnet; hp-phase, high-pressure phase; Hex.P, hexagonal aluminum phase; Jd, jadeite; Lgt, lingunite; LDA, local density approximation functional; Maj, majorite; mLd, phase with the modified ludwigite-type structure; NAL, new hexagonal alumina phase; Ol, olivine; Per, periclase; Pv, MgSiO3 with the perovskite-type structure; PPv, MgSiO3 with the post-perovskite-type structure; Rwd, ringwoodite; Sp, spinel.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iskrina, A.V., Bobrov, A.V. & Spivak, A.V. Post-Spinel Phases in the Earth’s Mantle. Geochem. Int. 60, 311–324 (2022). https://doi.org/10.1134/S0016702922040024
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702922040024