Abstract
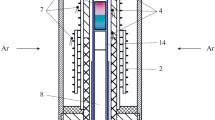
Water diffusion is one of the most important characteristics of many processes dealt with in magmatic geochemistry, petrology, and volcanology. We have experimentally examined water diffusion in Fe-free andesite and basalt melts (stoichiometric mixtures of the minerals albite + diopside + wollastonite) at 3 and 100 MPa, 1300×C, up to approximately 4 wt % water in the melts, and a total (lithostatic) pressure of 100 MPa on a high gas pressure apparatus equipped with a unique internal device. The experiments were conducted simultaneously with the use of two different methods: diffusion hydration and couples. Water solubility in the melts and water concentrations along the diffusion profiles (C H 2 O) were determined by quantitative IR microspectroscopy, using the Beer-Lambert law. A structural chemical model is proposed for calculating and predicting the concentration dependence of the molar absorption coefficient of the hydroxyl group and water molecules in andesite and basalt glasses. The diffusion coefficients of water (D H 2 O) are derived by the mathematical analysis of concentration profiles and the analytical solution of the second Fick diffusion law. Preliminary results indicated D H 2 O is roughly one order of magnitude higher in basaltic melts than in andesitic ones (at the same temperatures and P H 2 O) and significantly (exponentially) increases with increasing water concentrations in andesitic and basaltic melts. The newly obtained experimental data are proved to be fully consistent with the results obtained on the D H 2 O dependence on C H 2 O in melts of acid rocks (rhyolite and obsidian). The derived quantitative dependence between D H 2 O and melt viscosity is used to develope principles of a new method for predicting and calculating the temperature, concentration, and pressure dependences of D H 2 O in magmatic melts of the of acid-basic series (up to 3 wt % C H 2 O) at crustal T, P parameters.
Similar content being viewed by others
References
A. S. Chekhmir, A. G. Simakin, and M. B. Epel’baum, Dynamic Phenomena in Fluid-Magma Systems (Nauka, Moscow, 1991) [in Russian].
E. B. Watson, “Diffusion in Volatile-Bearing Magmas,” in Reviews in Mineralogy, Ed. by M. R. Carroll and J. R. Holloway (MSA, Washington, 1994), Vol. 30, pp. 371–411.
E. S. Persikov, P. G. Bukhtiyarov, and A. N. Nekrasov, “Experimental Study of the Diffusion of Major Components in the Melts of Albite-Diopside-H2O System at High Pressures,” in Proceedings of the 15th Russian Conference on Experimental Mineralogy (Geoprint, Syktyvkar, 2005) [in Russian].
H. R. Shaw, “Diffusion of H2O in Granitic Liquids; Part I. Experimental Data; Part II. Mass Transfer in Magma Chambers,” in Geochemical Transport and Kinetics, Ed. by A. W. Hofmann et al., (Carnegie Inst., Washington, 1974), Vol. 634, pp. 139–170.
D. B. Delaney and J. L. Karsten, “Ion Microprobe Studies of Water in Silicate Melts: Concentration-Dependent Diffusion in Obsidian,” Earth Planet. Sci. Lett. 52, 191–202 (1981).
J. L. Karsten, J. R. Holloway, and D. B. Delaney, “Ion Microprobe Studies of Water in Silicate Melts: Temperature-Dependent Diffusion in Obsidian,” Earth Planet. Sci. Lett. 59, 420–428.
K. E. Lapham, J. R. Holloway, and D. B. Delaney, “Diffusion of H2O in Obsidian at Elevated Temperatures and Pressures,” J. Non-Cryst. Solids 67, 179–191 (1984).
A. S. Chekhmir and M. B. Epel’baum, “Diffusion in Magmatic Melts,” in Physical Chemistry of Magmas. Advances in Physical Geochemistry, Ed. by L. L. Perchuk and I. Kushiro (Springer, New York, 1991), Vol. 9, pp. 99–119.
Y. Zhang and E. M. Stolper, “Water Diffusion in a Basaltic Melt,” Nature 351, 306–309 (1991).
E. S. Persikov, V. A. Zharikov, P. G. Bukhtiyarov, and S. F. Pol’skoy, “The Effect of Volatiles on the Properties of Magmatic Melts,” Eur. J. Mineral. 2, 621–642 (1990).
Y. Zhang, E. M. Stolper, and G. J. Wassenrburg, “Diffusion of Water in Rhyolitic Glasses,” Geochim. Cosmochim. Acta 55, 441–456 (1991).
H. Behrens and M. Nowak, “The Mechanisms of Water Diffusion in Polymerized Silicate Melts,” Contrib. Mineral. Petrol. 126, 377–385 (1997).
R. H. Doremus, “Diffusion of Water in Rhyolite Glass: Diffusion-Reaction Model,” J. Non-Cryst. Solids 261, 101–107 (2000).
Y. Zhang and H. Behrens, “H2O Diffusion in Rhyolitic Melts and Glasses,” Chem. Geol. 169, 243–262 (2000).
H. Behrens, Y. Zhang, and Z. Xu, “H2O Diffusion in Dacitic and Andesitic Melts,” Geochim. Cosmochim. Acta 68, 5139–5150 (2004).
S. Chakraborty, “Diffusion in Silicate Melts, Structure, Dynamics, and properties of Silicate Melts,” in Reviews in Mineralogy, Ed. by J. F. Stebbins (MSA, Washington, 1995), Vol. 32, 411–503.
E. S. Persikov, E. M. Stolper, S. Newman, et al., “Water Diffusion in Silicate Melts. Unsolved Problems and Possible Ways to Solve Some of Them,” in 11th International Conference on Experimental Mineralogy, Petrology and Geochemistry. EMPG-XI (Bristol, 2006), p. 58.
Y. Zhang, “H2O in Rhyolitic Glasses and Melts: Measurement, Speciation, Solubility, and Diffusion,” Rev. Geophys. 37, 493–516 (1999).
E. V. Kislov, Ioko-Dovyren Layered Massif (Buryatsk. Nauchn. Izd., Ulan-Ude, 1998).
E. S. Persikov, “The Viscosity of Magmatic Liquids: Experiment, Generalized Patterns. A Model for Calculation and Prediction. Applications,” in Physical Chemistry of Magmas. Advances in Physical Geochemistry, Ed. by L. L. Perchuk and I. Kushiro (Springer, New York, 1991), Vol. 9, pp. 1–40.
E. S. Persikov and P. G. Bukhtiyarov, “Unique Gas High Pressure Apparatus to Study Fluid-Melts and Fluid-Solid-Melts Interaction with Any Fluid Composition at the Temperature up to 1400°C and at the Pressures up to 5 Kbars,” J. Conf. Abs. 7(1), 85 (2002).
E. S. Persikov and P. G. Bukhtiyarov, “Experimental Study of Influence of Lithostatic and Water Pressure on Viscosity of Silicate and Magmatic Melts. New Structural-Chemical Model for Calculation and Prediction of their Viscosity,” in Experimental Mineralogy, Some Results on the Turn of Century, Ed. by V. A. Zharikov and V. V. Fed’kin (Nauka, Moscow, 2004), Vol. 1, pp. 103–122 [in Russian].
C. W. Mandeville, J. D. Webster, M. J. Rutherford, et al., “Determination of Molar Absorptivities for Infrared Absorption Bands of H2O in Andesitic Glasses,” Am. Mineral. 87, 813–821 (2002).
S. Ohlhorst, H. Behrens, and F. Holtz, “Compositional Dependence of Molar Absorptivities of Near-Infrared OH- and H2O Bands in Rhyolitic to Basaltic Glasses,” Chem. Geol. 174, 5–20 (2001).
L. A. Silver, P. D. Ihinger, and E. M. Stolper, “The Influence of Bulk Composition on the Speciation of Water in Silicate Glasses,” Contrib. Mineral. Petrol. 104, 142–162 (1990).
S. Yamashita, T. Kitamura, and M. Kusakabe, “Infrared Spectroscopy of Hydrous Glasses of Arc Magma Compositions,” Geochem. J. 31, 169–174 (1997).
R. F. Bartholomew, B. L. Butler, H. L. Hoover, and C. K. Wu, “Infrared Spectra of a Water-Containing Glass,” J. Am. Ceram. Soc. 63, 481–485 (1980).
J. E. Dixon, E. M. Stolper, and J. R. Holloway, “An Experimental Study of Water and Carbon Dioxide Solubilities in Mid-Ocean Ridge Basaltic Liquids. Part I: Calibration and Solubility Model,” J. Petrol. 36, 1607–1631 (1995).
J. Acocella, M. Tomozawa, and E. B. Watson, “The Nature of Dissolved Water in Sodium Silicate Glasses and Its Effect on Various Properties,” J. Non-Crystal. Solids 65, 355–372 (1984).
E. S. Persikov, Viscosity of Magmatic Melts (Nauka, Moscow, 1984) [in Russian].
E. S. Persikov, “The Viscosity of Model and Magmatic Melts under the P-T Parameters of the Earth’s Crust and Upper Mantle,” Russ. J. Geol. Geophys. 39, 1780–1792 (1998).
A. Acosta-Vigil, D. London, and G. B. Morgan, VI, “Contrasting Interactions of Sodium and Potassium with H2O in Haplogranitic Liquids and Glasses at 200 MPa from Hydration-Diffusion Experiments,” Contrib. Mineral. Petrol. 149, 276–287 (2005).
M. Nowak and H. Behrens, “The Speciation of Water in Haplogranitic Glasses and Melts Determined by in situ Near-Infrared Spectroscopy,” Geochim. Cosmochim. Acta 59, 3445–3450 (1995).
M. Nowak and H. Behrens, “An Experimental Investigation on Diffusion of Water in Haplogranitic Melts,” Contrib. Mineral. Petrol. 126, 365–376 (1997).
A. H. Shen and H. Keppler, “Infrared Spectroscopy of Hydrous Silicate Melts to 1000°C and 10 kbar: Direct Observation of H2O Speciation in a Diamond-Anvil Cell,” Am. Mineral. 80, 1335–1338 (1995).
J. R. Sowerby and H. Keppler, “Water Speciation in Rhyolitic Melt Determined by in-situ Infrared Spectroscopy,” Am. Mineral. 84, 1843–1849 (1999).
J. Crank, The Mathematics of Diffusion (Clarendon Press, Oxford, 1975).
F. Sauer and V. Freise, “Diffusion in binaren Gemischen mit Volumenanderung,” Z. Elektrochem. Angew. Phys. Chem. 66, 353–363 (1962).
E. S. Persikov, “Viscosity of Model and Magmatic Melts at the T-P Conditions of the Earth’s Crust and Upper Mantle,” Geologiya I Geofizika. 39(12) 1798–1804 (1998).
E. S. Persikov, “Structural Chemical Model to Calculate and Predict the Viscosity of Magmatic Melts in Full Range of Compositions and Conditions,” in General Assembly EGU-2007. Geophys. Res. Abstr. (EGU, Vienna, 2007), Vol. 9, A-02262, SRef-ID:1607-7962/gra/EGU2007-A-02262C.
E. S. Persikov, S. Neeman, P.G. Bukhtigarov, A.N. Nekrasov, E.M. Stolper, “Water diffusion in model and natural magmatic melts: Experimantal study of water diffusion in basic melts” (Chem. geol., unpublished, in review).
E. S. Persikov, P. G. Bukhtiyarov, S. Newman, and E. M. Stolper, “Water Diffusion in the Magmatic Melts at High Pressures. Principles of Structural-Chemical Model for Calculation and Forecasting,” http://www.scgis.ru/russian/cp1251/h-dgggms/1-2008/informbul-1-2008/magm-27.pdf
S. Newman, E. Persikov, P. Bukhtiyarov, and E. Stolper, “Experimental Study of the Dependence of Water Diffusion on Water Content in Haploandesitic Melts,” in Conference Series. 12th International Conference on Experimental Mineralogy, Petrology, and Geochemistry, EMPG, Ed. by J. Konzett (Univ. Press, Innsbruck, 2008), p. 71
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.S. Persikov, P.G. Bukhtiyarov, A.N. Nekrasov, 2010, published in Geokhimiya, 2010, Vol. 48, No. 3, pp. 227–239.
Rights and permissions
About this article
Cite this article
Persikov, E.S., Bukhtiyarov, P.G. & Nekrasov, A.N. Water diffusion in basalt and andesite melts under high pressures. Geochem. Int. 48, 213–225 (2010). https://doi.org/10.1134/S0016702910030018
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702910030018