Abstract
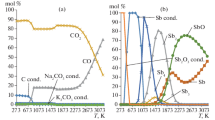
Distribution of plutonium and americium compounds in the combustion products of radioactive graphite in water vapor or air is analyzed. The study is carried out via thermodynamic analysis using the TERRA software package in a temperature range of 400–3200 K. It is revealed that all carbon in water vapor transitions into gas at temperatures above 900 K, and its transition temperature in air is 1000 K. Condensed plutonium compounds transform into vapor compounds in water vapor at temperatures above 1800 K and in air at 1700 K. Condensed americium compounds begin transforming into a vapor state at temperatures above 2000 K, and their transition temperature in air is 2200 K.













Similar content being viewed by others
REFERENCES
Nuclear Power Reactors in the World; https://www-pub.iaea.org/.
Rosatom: Power Generation; http://www.rosatom.ru.
S. A. Titov, N. M. Barbin, and A. M. Kobelev, “Analysis of Emergency Situations Related to Fires at Nuclear Power Plants," Pozharovzryvobezopasnost’ 30 (5), 66–75 (2021).
N. M. Barbin, S. A. Titov, and A. M. Kobelev, “Accidents that Occurred at Nuclear Power Plants in 1952–1972," IOP Conf. Ser.: Earth Environ. Sci. 666 (2), 022018 (2021); DOI: 10.1088/1755-1315/666/2/022018.
A. N. Dorofeev, E. A. Komarov, E. V. Zakharova, et al., “On Reactor Graphite Disposal," Radioactive Waste, No. 2 (7), 14–23 (2019); DOI: 10.25283/2587-9707-2019-2-18-30.
I. I. Linge and A. A. Abramova, Best Worldwide Practices for Decommissioning Nuclear Plants and Remediating Contaminated Territories (Rosatom, Moscow, 2017) [in Russian].
I. N. Bekman, Plutonium (Moscow State University, Moscow, 2010) [in Russian].
I. N. Bekman, Radiochemistry, Vol. 2: Radioactive Elements (Izdatel’ Markhotin P. Yu., Moscow, 2014) [in Russian].
V. A. Bazhenov, L. A. Buldakov, and I. Ya. Vasilenko, Harmful Chemicals (Khimiya, Leningrad, 1990) [in Russian].
M. A. Skachek, Radioactive Components of Nuclear Power Plants: Handling, Processing, and Localization (Izd. Dom MEI, Moscow, 2014) [in Russian].
L. L. Tsyganov, V. I. Khvostov, E. A. Komarov, et al., “The Problems of Utilizing Graphite of Stopped Graphite–Uranium Reactors," Izv. Tomsk. Politekh. Univ. 310 (2), 94–98 (2007) [Bull. Tomsk Polytech. Univ. 310 (2), 88–92 (2007)].
N. A. Vatolin, G. K. Moiseev, and B. G. Trusov, Thermodynamic Modeling in High Temperature Systems (Metallurgiya, Moscow, 1994) [in Russian].
N. M. Barbin, A. M. Kobelev, D. I. Terent’ev, and S. G. Alekseev, “Thermodynamic Modeling of Thermal Processes Involving Actinides (U, Am, Pu) in the Course of Heating Radioactive Graphite in Steam," Radiokhimiya 59 (5), 445–448 (2017) [Radiochemistry 59 (2), 507–511 (2017)].
G. V. Belov, Thermodynamic Modeling (Nauchnyi Mir, Moscow, 2002) [in Russian].
G. V. Belov and B. G. Trusov, Thermodynamic Modeling of Chemically Reacting Systems (Bauman Moscow State Technical University, Moscow, 2013) [in Russian].
Thermodynamic Properties of Individual Substances. Ivtanthermo Database; http://www.chem.msu.su/ rus/handbook/ivtan.
G. V. Belov, S. A. Dyachkov, P. R. Levashov, et al., “The IVTANTHERMO-Online Database for Thermodynamic Properties of Individual Substances," J. Phys.: Conf. Ser. 946, 012120 (2018); DOI: 10.1088/1742-6596/946/1/012120.
Thermodynamic Properties of Individual Substances: Digital Manual; http://twt.mpei.ac.ru/TTHB/2/ OIVT/IVTANThermo/Rus/index.htm#open1.
O. Dorofeeva, V. P. Novikov, and D. B. Neumann, “NIST-JANAF Thermochemical Tables. I. Ten Organic Molecules Related to Atmospheric Chemistry," J. Phys. Chem. Ref. Data 30 (2), 475–513 (2001); DOI: 10.1063/1.1364518.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizika Goreniya i Vzryva, 2022, Vol. 58, No. 4, pp. 24-31.https://doi.org/10.15372/FGV20220403.
Rights and permissions
About this article
Cite this article
Barbin, N.M., Kobelev, A.M., Titov, S.A. et al. Thermodynamic Analysis of Compositions of Combustion Products of Radioactive Graphite in Water Vapor or Air. Combust Explos Shock Waves 58, 415–421 (2022). https://doi.org/10.1134/S0010508222040037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0010508222040037