Abstract
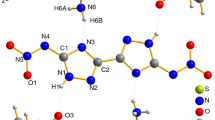
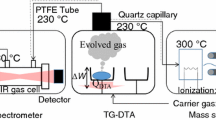
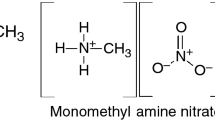
Despite significant progress in studying thermal decomposition of ammonium dinitramide (ADN), the kinetics of the process at the level of elementary stages has not been adequately understood. The aim of this review is to summarize various published data, which are of interest for studying and simulating the processes of thermal decomposition and combustion of ADN. Considerable attention is paid to physical and chemical properties of ADN, dinitramide and its anion N(NO2) -2 , which play a key role in ADN decomposition. Various paths of decomposition of ADN, dinitramide, and N(NO2) -2 are discussed. Results illustrating alternative points of view on the decomposition process are presented.
Similar content being viewed by others
References
O. A. Luk’yanov, V. P. Gorelik, and V. A. Tartakovsky, “Dinitramide and Its Salts. Communication 1. Obtaining Dinitramide Salts in the Reaction of Decyanethylization of N, N-dinitro-ß-amynopropyonitrile,” Izv. Akad. Nauk, Ser. Khim., No. 1, 94–97 (1994).
O. A. Luk’yanov, Yu. V. Konnova, T. A. Klimova, and V. A. Tartakovsky, “Dinitramide and Its Salts. Communication 2. Dinitramide in Direct and Reverse Michael-Type Reactions,” Izv. Akad. Nauk, Ser. Khim., No. 7, 1264–1266 (1994).
O. A. Luk’yanov, O. V. Anikin, V. P. Gorelik, and V. A. Tartakovsky, “Dinitramide and Its Salts. Communication 3. Dinitramide Salts with Metals,” Izv. Akad. Nauk, Ser. Khim., No. 9, 1546–1549 (1994).
V. A. Shlyapochnikov, N. O. Cherskaya, O. A. Luk’yanov, V. P. Gorelik, and V. A. Tartakovsky, “Dinitramide and Its Salts. Communication 4. Molecular Structure of Dinitramide,” Izv. Akad. Nauk, Ser. Khim., No. 9, 1610–1613 (1994).
O. A. Luk’yanov, N. I. Shlykova, and V. A. Tartakovsky, “Dinitramide and Its Salts. Communication 5. Alkylation of Dinitramide and Its Salts,” Izv. Akad. Nauk, Ser. Khim., No. 10, 1775–1778 (1994).
O. A. Luk’yanov, A. R. Agevnin, A. A. Leichenko, N. M. Seregina, and V. A. Tartakovsky, “Dinitramide and Its Salts. Communication 6. Dinitramide Salts with Ammonium Bases,” Izv. Akad. Nauk, Ser. Khim., No. 1, 113–117 (1995).
S. Venkatachalam, G. Santhosh, and K. N. Ninan, “An Overview on the Synthetic Routes and Properties of Ammonium Dinitramide (ADN) and Other Dinitramide Salts,” Propell., Explos., Pyrotech. 29 (3), 178–187 (2004).
O. A. Luk’yanov and V. A. Tartakovsky, “Synthesis and Characterization of Dinitramidic Acid and Its Salts,” in Progress in Astronautics and Aeronautics, Vol. 185: Solid Propellant Chemistry, Combustion, and Motor Interior Ballistics, Ed. by V. Yang (2000), Ch. 1.8, pp. 207–220.
K. O. Christe, W. W. Wilson, M. A. Petrie, et al., “The Dinitramide Anion, N(NO2)-2,” Inorg. Chem. 35, 5068–5071 (1996).
V. V. Gidaspov, I. V. Tselinskii, V. V. Mel’nikov, et al., “Crystalline Molecular Structure of Dinitramide Salts and Its Acid–Base Properties,” Zh. Obsch. Khim. 65 (6), 995–1002 (1995).
H. F. R. Schoyer, A. J. Schnork, P. A. Korting, et al., “High-Performance Propellants Based on Hydrazinium Nitroformate,” J. Propuls. Power 11 (4), 856–869 (1995).
H. Bathelt and F. Volk, The ICT—Thermochemical Data Base (Fraunhofer Institut fur Chemische Technologie, 1997).
H. Ostmark, U. Bemm, A. Langlet, R. Sanden, and N. Wingborg, “The Properties of Ammonium Dinitramide (ADN): Part 1, Basic Properties and Spectroscopic Data,” J. Energ. Mater. 18, 123–138 (2000).
T. S. Kon’kova, Yu. N. Matyushin, E. A. Miroshnichenko, and A. B. Vorob’ev, “Thermochemical Properties of Dinitramidic Acid Salts,” Izv. Akad. Nauk, Ser. Khim., No. 10, 1958–1965 (2009).
S. Lobbecke, T. Keicher, H. Krause, and A. Pfeil, “The New Energetic Material Ammonium Dinitramide and Its Thermal Decomposition,” Solid State Ionics 101-103, 945–951 (1997).
J. C. Bottaro, P. E. Penwell, and R. J. Schmitt, “1,1,3,3-Tetraoxo-1,2,3-Triazapropene Anion, a New Oxy Anion of Nitrogen: The Dinitramide Anion and Its Salts,” J. Amer. Chem. Soc. 119, 9405–9410 (1997).
D. E. C. Jones, Q. S. M. Kwok, M. Vachon, C. Badeen, and W. Ridley, “Characterization of ADN and ADNBased Propellants,” Propell., Explos., Pyrotech. 30 (2), 140–147 (2005).
A. S. Tompa, “Thermal Analysis of Ammonium Dinitramide (ADN),” Thermochim. Acta 357–358, 177–193 (2000).
Z. Pak, “Some Ways to Higher Environmental Safety of Solid Rocket Propellant Application,” in 29th Joint Propulsion Conference and Exhibit, Monterey, June 28–30, 1993, CAAIAA Paper No. 93-1755 (1993).
N. Wingborg, “Ammonium Dinitramide–Water: Interaction and Properties,” J. Chem. Eng. Data 51, 1582–1586 (2006).
R. Yang, P. Thakre, and V. Yang, “Thermal Decomposition and Combustion of Ammonium Dinitramide (Review),” Fiz. Goreniya Vzryva 41 (6), 54–59 (2005) [Combust., Expl., Shock Waves 41 (6), 657–679 (2005)].
T. P. Russel, G. J. Piermarini, S. Block, and P. J. Miller, “Pressure, Temperature Reaction Phase Diagram for Ammonium Dinitramide,” J. Phys. Chem. 100 (8), 3248–3251 (1996).
G. B. Manelis, “Thermal Decomposition of Dinitramide Salt,” in Pyrotechnics: Basic Principles, Technology, Application, 26th Int. Annu. Conf. of ICT (Karlsruhe, 1995), pp. 15.1–15.17.
A. N. Pavlov, V. N. Grebennikov, L. D. Nazina, et al., “Thermal Decomposition of Ammonium Dinitramide and Mechanism of Anomalous Decomposition of Dinitramide Salts,” Izv. Akad. Nauk, Ser. Khim., No. 1, 50–54 (1999).
A. I. Kazakov, Yu. I. Rubtsov, G. B. Manelis, and L. P. Andrienko, “Kinetics of Dinitramide Decomposition. Communication 1. Decomposition of Different Forms of Dinitramide,” Izv. Akad. Nauk, Ser. Khim., No. 12, 2129–2133 (1997).
H. H. Michels and J. A. Montgomery, “On the Structure and Thermochemistry of Hydrogen Dinitramide,” J. Phys. Chem. 97 (25), 6602–6606 (1993).
P. Politzer and J. M. Seminario, “Computational Study of the Structure of Dinitraminic Acid, HN(NO2)2, and the Energetics of Some Possible Decomposition Steps,” Chem. Phys. Lett. 216 (3–6), 348–352 (1993).
A. M. Mebel, M. C. Lin, K. Morokuma, and C. F. Melius, “Theoretical Study of the Gas-Phase Structure, Thermochemistry and Decomposition Mechanisms of NH4NO2 and NH4N(NO2)2,” J. Phys. Chem. 99 (18), 6842–6848 (1995).
P. Politzer, J. M. Seminario, and M. C. Concha, “Energetics of Ammonium Dinitramide Decomposition Steps,” J. Mol. Struct. (Theochem) 427, 123–129 (1998).
S. Alavi and D. L. Thompson, “Decomposition Pathways of Dinitramic Acid and the Dinitramide Ion,” J. Chem. Phys. 119 (1), 232–240 (2003).
M. A. Bohn and M. E. Grillo, “Quantum Mechanical Calculations Used to Reveal Decomposition Ways of Ammonium Dinitramide (ADN),” in 37th Int. Annu. Conf. of ICT (Karlsruhe, 2006), pp. 74.1–74.17.
M. Rahm and T. Brinck, “Dinitraminic Acid (HDN) Isomerization and Self-Decomposition Revised,” Chem. Phys. 348, 53–60 (2008).
V. A. Shlyapochnikov, G. I. Oleneva, N. O. Cherskaya, et al., “Dinitramide and Its Salts. Communication 7. Spectra and Structure of Dinitramide Salts,” Izv. Akad. Nauk, Ser. Khim., No. 8, 1508–1511 (1995).
V. A. Shlyapochnikov, M. A. Tafipolsky, I. V. Tokmakov, et al., “On the Structure and Spectra of Dinitramide Salts,” J. Mol. Struct. 559, 147–166 (2001).
R. Gilardi, J. Flippen-Anderson, C. George, and R. J. Butcher, “A New Class of Flexible Energetic Salts: The Crystal Structures of the Ammonium, Lithium, Potassium, and Cesium Salts of Dinitramide.” J. Amer. Chem. Soc. 119, 9411–9416 (1997).
F. I. Dubovitskii, N. I. Golovina, A. N. Pavlov, and L. O. Atovmyan, “Structural Features of Dinitramide Salts with Alkali Metals,” Dokl. Akad. Nauk 355 (2), 200–202 (1997).
J. Cui, J. Han, J. Wang, and R. Huang, “Study on the Crystal Structure and Hygroscopicity of Ammonium Dinitramide,” J. Chem. Eng. Data 55, 3229–3234 (2010).
R. J. Schmitt, M. Krempp, and V. M. Bierbaum, “Gas Phase Chemistry of Dinitramide and Nitroacetylide Ions,” J. Mass Spectrom. Ion Proces. 177, 621–632 (1992).
R. J. Doyle, “Sputtered Ammonium Dinitramide: Tandem Mass Spectrometry of a New Ionic Nitramide,” Org. Mass Spectrom. 28 (2), 83–91 (1993).
S. Alavi and D. L. Thompson, “Proton Transfer in Gas-Phase Ammonium Dinitramide Clusters,” J. Chem. Phys. 118 (6), 2599–2605 (2003).
M. J. Rossi, J. C. Bottaro, and D. F. Mc Millen, “The Thermal Decomposition of the New Energetic Material Ammonium Dinitramide (NH4N(NO2)2) inRelation to Nitramide (NH2NO2) and NH4NO3,” Int. J. Chem. Kinet. 25, 549–570 (1993).
A. Snelson and A. J. Tulis, “Vaporization of NH4N(NO2)2 and Tentative Identification of HN(NO2)2 by IR Matrix Isolation Spectroscopy,” in Proc. 19th Int. Pyrotechnics Seminar, Christchurch, New Zeland, February 20–25, 1994, pp. 531–544.
F. I. Dubovitskii, G. A. Voklov, V. N. Grebennikov, et al., “Thermal Decomposition of the Potassium Salt of Dinitramide in the Liquid State,” Dokl. Akad. Nauk 347 (6), 763–765 (1996).
F. I. Dubovitskii, G. A. Voklov, V. N. Grebennikov, et al., “Thermal Decomposition of the Potassium Salt of Dinitramide in the Solid State,” Dokl. Akad. Nauk 348 (2), 205–206 (1996).
S. B. Babkin, A. N. Pavlov, and G. M. Nazin, “Anomalous Decomposition of Dinitramide Salts with Metals in the Solid State,” Izv. Akad. Nauk, Ser. Khim., No. 11, 1947–1950 (1997).
A. N. Pavlov and G. M. Nazin, “Decomposition Mechanism of Dinitramide Salts. Anomalous Decomposition of Dinitramide Metal Salts and Ammonium Salt in the Solid Phase,” in: Energetic Materials, Production, Processing and Characterization: 29th Int. Annu. Conf. of ICT (Karlsruhe, 1998), Paper 25, pp. 1–14.
A. N. Pavlov and G. M. Nazin, “Mechanism of Decomposition of Onium Salts of Dinitramide,” Izv. Akad. Nauk, Ser. Khim., No. 11, 1951–1953 (1997).
A. I. Kazakov, Yu. I. Rubtsov, G. B. Manelis, and L. P. Andrienko, “Kinetics of Dinitramide Decomposition. Communication 2. Kinetics of Dinitramide Interaction with Products of Decomposition and Other Components of the Solution,” Izv. Akad. Nauk, Ser. Khim., No. 1, 41–47 (1998).
A. I. Kazakov, Yu. I. Rubtsov, L. P. Andrienko, and G. B. Manelis “Kinetics of Dinitramide Decomposition. Communication 3. Kinetics of Heat Release during Thermal Decomposition of the Ammonium Salt of Dinitramide in the Liquid Phase,” Izv. Akad. Nauk, Ser. Khim., No. 3, 395–401 (1998).
A. I. Kazakov, Y. I. Rubtsov, and G. B. Manelis, “Kinetics and Mechanism of Thermal Decomposition of Dinitramide,” Propell., Explos., Pyrotech. 24, 37–42 (1999).
T. B. Brill, P. J. Brush, and D. G. Patil, “Thermal Decomposition of Energetic Materials 58. Chemistry of Ammonium Nitrate and Ammonium Dinitramide near the Burning Surface Temperature,” Combust. Flame 92 (1-2), 178–186 (1993).
J. C. Oxley, J. L. Smith, W. Zheng, et al., “Thermal Decomposition Studies on Ammonium Dinitramide (ADN) and 15N and 2H Isotopomers,” J. Phys. Chem. A 101 (31), 5646–5652 (1997).
S. Vyazovkin and C. A. Wight, “Ammonium Dinitramide: Kinetics and Mechanism of Thermal Decomposition,” J. Phys. Chem. A 101 (31), 5653–5658 (1997).
S. Vyazovkin and C. A. Wight, “Thermal Decomposition of Ammonium Dinitramide at Moderate and High Temperatures,” J. Phys. Chem. A 101 (39), 7217–7221 (1997).
H. Matsunaga, H. Habu, and A. Miyake, “Thermal Behavior of New Oxidizer Ammonium Dinitramide,” J. Therm. Anal. Calorim. 111, 1183–1188 (2013).
H. Matsunaga, H. Habu, and A. Miyake, “Influences of Aging on Thermal Decomposition Mechanism of High Performance Oxidizer Ammonium Dinitramide,” J. Therm. Anal. Calorim. 113, 1384–1394 (2013).
K. Fujisato, H. Habu, and K. Hori, “Condensed Phase Behavior in the Combustion of Ammonium Dinitramide,” Propell., Explos., Pyrotech. 39 (5), 714–722 (2014).
I. B. Mishra and T. P. Russell, “Thermal Stability of Ammonium Dinitramide,” Thermochim. Acta 384, 47–56 (2002).
A. B. Andreev, O. V. Anikin, A. P. Ivanov, et al., “Stabilization of the Ammonium Salt of Dinitramide in the Liquid State,” Izv. Akad. Nauk, Ser. Khim., No. 12, 2006–2008 (2000).
G. Santhosh, S. Venkatachalam, K. Krishnan, et al., “A Thermogravimetric Study on the Thermal Decomposition of Ammonium Dinitramide (ADN)—Potassium Dinitramide (KDN) Mixtures,” in Proc. 34th Int. Annu. Conf. of ICT (Karlsruhe, 2003), pp. 1–9.
R. S. Zhu, H.-L. Chen, and M. C. Lin, “Mechanism and Kinetics for Ammonium Dinitramide (ADN) Sublimation: A First-Principles Study,” J. Phys. Chem. A 116, 10836–10841 (2012).
L. V. Gurvich, I. V. Veits, V. A. Medvedev, et al., Thermodynamic Properties of Individual Substances (Nauka, Moscow, 1978), Vol. 1, Book 2 [in Russian].
J. Park, D. Chakraborty, and M. C. Lin, “Thermal Decomposition of Gaseous Ammonium Dinitramide at Low Pressure: Kinetic Modeling of Product Formation with ab Initio MO / c VRRKM Calculations,” in Proc. 27th Symp. (Int.) on Combustion (The Combustion Inst., 1998), pp. 2351–2357.
V. P. Sinditskii, V. Y. Egorshev, A. I. Levshenkov, and V. V. Serushkin, “Combustion of Ammonium Dinitramide. Part 2: Combustion Mechanism,” J. Propuls. Power 22 (4), 777–785 (2006).
A. A. Zenin, V. M. Puchkov, and S. V. Finjakov, “Physics of ADN Combustion,” in 37th Aerospace Sciences Meeting and Exhibit, Reno, January 11–14, 1999, AIAA Paper No. 1999-0595 (1999).
V. P. Sinditskii, V. Yu. Egorshev, V. V. Serushkin, and S. A. Filatov, “Combustion of Energetic Materials Controlled by Condensed-Phase Reactions,” Fiz. Goreniya Vzryva 48 (1), 89–109 (2012) [Combust., Expl., Shock Waves 48 (1), 81–99 (2012)].
A. Hahma, H. Edvinsson, and H. Östmark, “The Properties of Ammonium Dinitramine (ADN): Part 2: Melt Casting,” J. Energ. Mater. 28, 114–138 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.E. Ermolin, V.M. Fomin.
Published in Fizika Goreniya i Vzryva, Vol. 52, No. 5, pp. 79–101, September–October, 2016.
Rights and permissions
About this article
Cite this article
Ermolin, N.E., Fomin, V.M. On the mechanism of thermal decomposition of ammonium dinitramide (review). Combust Explos Shock Waves 52, 566–586 (2016). https://doi.org/10.1134/S0010508216050087
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0010508216050087