Abstract
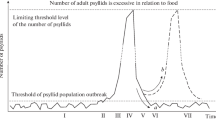
A model scenario of a drastic increase in the number of a phytophagous species (jumping plant lice) at the expense of primary and secondary Encyrtidae parasites (a large family of parasitic microwasps) has been developed based on the case study of changes in the local density of a Psyllidae species in Australia. A phenomenological model differentially describes the reproduction efficiency in several ranges of the population state. A continuous-event structure is proposed where the rate of decrease in the abundance of generations is uneven at different developmental stages of the insect with an incomplete metamorphosis and the points where it changes are determined by the state of internal variables in the auxiliary equation for the continuous system. A spontaneous time-limited local outbreak occurs after overcoming the threshold equilibrium in the iterative dynamic system, which reduces the effect of normal regulatory mechanisms of psyllid reproduction and changes the rate of the decrease in the abundance of generations. The method of supplementing the right-hand part of the first equation by special functional with a limited range of values simulates a drastic decrease in survival with depletion of resources. This modification causes a backward tangent bifurcation. Several generations after the bifurcation, the population switches to the mode of fluctuations without any pronounced cyclic component, which is common for small insects with a low abundance.
Similar content being viewed by others
References
G. F. Gauze and A. A. Vitt, Izv. Akad. Nauk SSSR 10, 1551 (1934).
A. D. Bazykin and A. I. Khibnik, Biofizika 26 (5), 851 (1981).
M. M. Gonik, A. E. Bobyrev, V. A. Burmensky, et al., Biophysics (Moscow) 52 (4), 445 (2007).
P. Mulock and E. Christiansen, For. Ecol. Manag. 14 (2), 125 (1986).
D. Ludwig, D. D. Jones, and C. S. Holling, J. Anim. Ecol. 47 (1), 315 (1978).
L. R. Clark, Austral. J. Zool. 12 (3), 362 (1964).
D. R. Gray, et al., For. Ecol. Manag 127 (1), 217 (2000).
J. Berry, Biosecurity 68, 18 (2006).
L. R. Clark and M. J. Dallwitz, Austral. J. Zool. 23 (4), 523 (1975).
E. A. Kriksunov and M. A. Snetkov, Dokl. Akad. Nauk SSSR 253 (3), 759 (1980).
L. V. Nedorezon and Yu. V. Utyupin, Continuous–Discrete Models of Population Dynamics (GPTNB SO RAN, Novosibirsk, 2011) [in Russian].
Y. Kolesov and Y. Senichenkov, in Proc. 8th EUROSIM Congr. on Modelling and Simulation (2013), p. 294.
T. H. Keitt, M. A. Lewis, and R. D. Holt, Am. Nat. 157 (2), 203 (2001).
P. C. Tobin, Popul. Ecol. 51, 373 (2009).
M. J. Feigenbaum, Commun. Math. Phys. 77 (1), 65 (1980).
C. Grebogi, E. Ott, and J. Yorke, Phys. Rev. Lett. 56 (10), 1011 (1986).
J. Graczyk, D. Sands, and G. Swiatek, Ann. Math. 159, 725 (2004).
G. B. Astafev, A. A. Koronovski, and A. E. Hramov, Techn. Phys. Lett. 29 (11), 923 (2003).
H. Kantz and P. Grassberger, Physica D: Nonlin. Phenom. 17 (1), 75 (1985).
L. Hong and Ji. Xu, Nonlin. Dynam. 32 (4), 371 (2003).
C. Li, Nonlin. Anal. Model. Control 18 (1), 66 (2013).
A. Y. Perevaryukha, J. Comput. Sys. Sci. Int. 50 (3), 491 (2011).
J. Dushoff, et al., J. Math. Biol 36, 227 (1998).
P. Coullet and C. Tresser, J. de Physique 39 (8), 25 (1978).
M. J. Ferigenbaum, J. Stat. Phys. 21 (6), 669 (1979).
P. Barbosa, D. Letourneau, and A. Agrawal, Insect Outbreaks Revisited (Wiley, Oxford, 2012).
S. V. Pushkin, Ross. Zh. Biol. Invazii 1 (1), 42 (2008).
E. V. Aistova and V. G. Bezborodov, Chteniya Pamyati A. I. Kurentsova 26, 144 (2015).
A. G. Moseyko, Entomol. Rev. 93 (2), 208 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.Yu. Perevaryukha, 2016, published in Biofizika, 2016, Vol. 61, No. 2, pp. 395–404.
Rights and permissions
About this article
Cite this article
Perevaryukha, A.Y. An iterative continuous-event model of the population outbreak of a phytophagous Hemipteran. BIOPHYSICS 61, 334–341 (2016). https://doi.org/10.1134/S0006350916020147
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350916020147