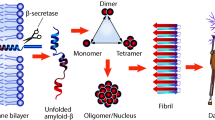
Abstract
Progression of Alzheimer’s disease is accompanied by the appearance of extracellular deposits in the brain tissues of patients with characteristic supramolecular morphology (amyloid plaques) the main components of which are β-amyloid isoforms (Aβ) and biometal ions (zinc, copper, iron). For nearly 40 years and up to the present time, the vast majority of experimental data indicate critical role of formation and accumulation of amyloid plaques (cerebral amyloidogenesis) in pathogenesis of Alzheimer’s disease, however, nature of the molecular agents that initiate cerebral amyloidogenesis, as well as causes of aggregation of the native Aβ molecules in vivo remained unknown for a long time. This review discusses the current level of fundamental knowledge about the molecular mechanisms of interactions of zinc ions with a number of Aβ isoforms present in amyloid plaques of the patients with Alzheimer’s disease, and also shows how this knowledge made it possible to identify driving forces of the cerebral amyloidogenesis in Alzheimer’s disease and made it possible to determine fundamentally new biomarkers and drug targets as part of development of innovative strategy for diagnosis and treatment of Alzheimer’s disease.




Similar content being viewed by others
Abbreviations
- Aβ:
-
amyloid beta
- Aβ(i-j):
-
a continuous linear fragment starting from i-th position to j-th position of the human Aβ amino acid sequence, 1DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA42 (single-letter amino acid code)
- AD:
-
Alzheimer’s disease
- CA:
-
cerebral amyloidogenesis in Alzheimer’s disease
- HAEE:
-
tetrapeptide acetyl-HAEE-NH2
- isoD7:
-
isomerized D7
- isoD7-Aβ:
-
isoD7-bearing amyloid beta
- NMR:
-
nuclear magnetic resonance
- pS8:
-
phosphorylated serine residue at S8
- pS8-Aβ:
-
pS8-bearing amyloid beta
References
Gavrilova, S. I. (2007) Alzheimer’s Disease Pharmacotherapy [In Russian], Pul’s, Moscow, 360 p.
Alzheimer's Association (2020) 2020 Alzheimer’s disease facts and figures, Alzheimers Dement., 16, 391-460, https://doi.org/10.1002/alz.12068.
Editorial (2014) G8 dementia summit: a chance for united action, Lancet Neurol., 13, 1, https://doi.org/10.1016/S1474-4422(13)70275-8.
URL: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on January 13, 2023).
Yiannopoulou, K. G., and Papageorgiou, S. G. (2020) Current and future treatments in Alzheimer disease: an update, J. Central Nerv. System Dis., 12, https://doi.org/10.1177/1179573520907397.
Long, J. M., and Holtzman, D. M. (2019) Alzheimer’s disease: an update on pathobiology and treatment strategies, Cell, 179, 312-339, https://doi.org/10.1016/j.cell.2019.09.001.
Querfurth, H. W., and LaFerla, F. M. (2010) Alzheimer’s disease, N. Engl. J. Med., 362, 329-344, https://doi.org/10.1056/NEJMra0909142.
Ghosh, S., Durgvanshi, S., Agarwal, S., Raghunath, M., and Sinha, J. K. (2020) Current status of drug targets and emerging therapeutic strategies in the management of Alzheimer’s disease, Curr. Neuropharmacol., 18, 883-903, https://doi.org/10.2174/1570159X18666200429011823.
Cummings, J., Lee, G., Ritter, A., Sabbagh, M., and Zhong, K. (2020) Alzheimer’s disease drug development pipeline: 2020, Alzheimers Dement., 6, e12050, https://doi.org/10.1002/trc2.12050.
Jack, C. R., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S. B., Holtzman, D. M., Jagust, W., Jessen, F., Karlawish, J., Liu, E., Molinuevo, J. L., Montine, T., Phelps, C., Rankin, K. P., Rowe, C. C., Scheltens, P., Siemers, E., Snyder, H. M., Sperling, R., Elliott, C., Masliah, E., Ryan, L., and Silverberg, N. (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease, Alzheimers Dement., 14, 535-562, https://doi.org/10.1016/j.jalz.2018.02.018.
Jagust, W., Jack, C. R., Jr., Bennett, D. A., Blennow, K., Haeberlein, S. B., Holtzman, D. M., Jessen, F., Karlawish, J., Liu, E., Molinuevo, J. L., Montine, T., Phelps, C., Rankin, K. P., Rowe, C. C., Scheltens, P., Siemers, E., and Sperling, R. (2019) “Alzheimer’s disease” is neither “Alzheimer’s clinical syndrome” nor “dementia”, Alzheimers Dement., 15, 153-157, https://doi.org/10.1016/j.jalz.2018.11.002.
Serrano-Pozo, A., Frosch, M. P., Masliah, E., and Hyman, B. T. (2011) Neuropathological alterations in Alzheimer’s disease, Cold Spring Harb. Perspect. Med., 1, a006189, https://doi.org/10.1101/cshperspect.a006189.
Small, S. A., Schobel, S. A., Buxton, R. B., Witter, M. P., and Barnes, C. A. (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease, Nat. Rev. Neurosci., 12, 585-601, https://doi.org/10.1038/nrn3085.
Braak, H., Braak, E., and Bohl, J. (1993) Staging of Alzheimer-Related Cortical Destruction, Eur. Neurol., 33, 403-408, https://doi.org/10.1159/000116984.
Wahlund, L.-O., Julin, P., Lindqvist, J., and Scheltens, P. (1999) Visual assessment of medial temporal lobe atrophy in demented and healthy control subjects: correlation with volumetry, Psychiatry Res., 90, 193-199, https://doi.org/10.1016/S0925-4927(99)00016-5.
Hampel, H., Mesulam, M. M., Cuello, A. C., Farlow, M. R., Giacobini, E., Grossberg, G. T., Khachaturian, A. S., Vergallo, A., Cavedo, E., Snyder, P. J., and Khachaturian, Z. S. (2018) The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease, Brain, 141, 1917-1933, https://doi.org/10.1093/brain/awy132.
Polis, B., and Samson, A. O. (2019) A new perspective on Alzheimer’s disease as a brain expression of a complex metabolic disorder, Alzheimer’s Disease [Internet] (Wisniewski, T., ed.) Codon Publications, Brisbane (AU), https://doi.org/10.15586/alzheimersdisease.2019.ch1.
Karran, E., Mercken, M., and De Strooper, B. (2011) The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics, Nat. Rev. Drug Discov., 10, 698-712, https://doi.org/10.1038/nrd3505.
Selkoe, D. J., and Hardy, J. (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years, EMBO Mol. Med., 8, 595-608, https://doi.org/10.15252/emmm.201606210.
Golde, T. E., DeKosky, S. T., and Galasko, D. (2018) Alzheimer’s disease: the right drug, the right time, Science, 362, 1250-1251, https://doi.org/10.1126/science.aau0437.
Huang, L. K., Chao, S. P., and Hu, C. J. (2020) Clinical trials of new drugs for Alzheimer’s disease, J. Biomed. Sci., 27, 18, https://doi.org/10.1186/s12929-019-0609-7.
Masters, C. L., and Selkoe, D. J. (2012) Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer Disease, Cold Spring Harb. Perspect. Med., 2, a006262, https://doi.org/10.1101/cshperspect.a006262.
Wang, J., Gu, B. J., Masters, C. L., and Wang, Y.-J. (2017) A systemic view of Alzheimer disease – insights from amyloid-β metabolism beyond the brain, Nat. Rev. Neurol., 13, 612-623, https://doi.org/10.1038/nrneurol.2017.111.
Brothers, H. M., Gosztyla, M. L., and Robinson, S. R. (2018) The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer’s disease, Front. Aging Neurosci., 10, 118, https://doi.org/10.3389/fnagi.2018.00118.
Smith, L. M., and Strittmatter, S. M. (2017) Binding sites for amyloid-β oligomers and synaptic toxicity, Cold Spring Harb. Perspect. Med., 7, a024075, https://doi.org/10.1101/cshperspect.a024075.
Cohen, S. I. A., Linse, S., Luheshi, L. M., Hellstrand, E., White, D. A., Rajah, L., Otzen, D. E., Vendruscolo, M., Dobson, C. M., and Knowles, T. P. J. (2013) Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism, Proc. Natl. Acad. Sci. USA, 110, 9758-9763, https://doi.org/10.1073/pnas.1218402110.
Masters, C. L., Simms, G., Weinman, N. A., Multhaup, G., McDonald, B. L., and Beyreuther, K. (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome, Proc. Natl. Acad. Sci. USA, 82, 4245-4249, https://doi.org/10.1073/pnas.82.12.4245.
Mukherjee, S., Perez, K. A., Lago, L. C., Klatt, S., McLean, C. A., Birchall, I. E., Barnham, K. J., Masters, C. L., and Roberts, B. R. (2021) Quantification of N-terminal amyloid-β isoforms reveals isomers are the most abundant form of the amyloid-β peptide in sporadic Alzheimer’s disease, Brain Commun., 3, fcab028, https://doi.org/10.1093/braincomms/fcab028.
Walker, L. C. (2020) Aβ Plaques, Free Neuropathol., 1, 31, https://doi.org/10.17879/freeneuropathology-2020-3025.
Stewart, K. L., and Radford, S. E. (2017) Amyloid plaques beyond Aβ: a survey of the diverse modulators of amyloid aggregation, Biophys. Rev., 9, 405-419, https://doi.org/10.1007/s12551-017-0271-9.
Kollmer, M., Close, W., Funk, L., Rasmussen, J., Bsoul, A., Schierhorn, A., Schmidt, M., Sigurdson, C. J., Jucker, M., and Fändrich, M. (2019) Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer’s brain tissue, Nat. Commun., 10, 4760, https://doi.org/10.1038/s41467-019-12683-8.
Takano, K. (2008) Amyloid beta conformation in aqueous environment, Curr. Alzheimer Res., 5, 540-547, https://doi.org/10.2174/156720508786898424.
Eisenberg, D., and Jucker, M. (2012) The amyloid state of proteins in human diseases, Cell, 148, 1188-1203, https://doi.org/10.1016/j.cell.2012.02.022.
Jucker, M., and Walker, L. C. (2011) Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders, Ann. Neurol., 70, 532-540, https://doi.org/10.1002/ana.22615.
Soto, C., and Pritzkow, S. (2018) Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases, Nat. Neurosci., 21, 1332-1340, https://doi.org/10.1038/s41593-018-0235-9.
Jucker, M., and Walker, L. C. (2018) Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases, Nat. Neurosci., 21, 1341-1349, https://doi.org/10.1038/s41593-018-0238-6.
Craddock, T. J. A., Tuszynski, J. A., Chopra, D., Casey, N., Goldstein, L. E., Hameroff, S. R., and Tanzi, R. E. (2012) The zinc dyshomeostasis hypothesis of Alzheimer’s disease, PLoS One, 7, e33552, https://doi.org/10.1371/journal.pone.0033552.
Lovell, M. A., Robertson, J. D., Teesdale, W. J., Campbell, J. L., Markesbery, W. R. (1998) Copper, iron and zinc in Alzheimer’s disease senile plaques, J. Neurol. Sci., 158, 47-52, https://doi.org/10.1016/S0022-510X(98)00092-6.
Bush, A. I., Pettingell, W. H., Multhaup, G., d Paradis, M., Vonsattel, J. P., Gusella, J. F., Beyreuther, K., Masters, C. L., and Tanzi, R. E. (1994) Rapid induction of Alzheimer A beta amyloid formation by zinc, Science, 265, 1464-1467, https://doi.org/10.1126/science.8073293.
Miller, Y., Ma, B., and Nussinov, R. (2010) Zinc ions promote Alzheimer Abeta aggregation via population shift of polymorphic states, Proc. Natl. Acad. Sci. USA, 107, 9490-9495, https://doi.org/10.1073/pnas.0913114107.
Miller, L. M., Wang, Q., Telivala, T. P., Smith, R. J., Lanzirotti, A., and Miklossy, J. (2006) Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer’s disease, J. Struct. Biol., 155, 30-37, https://doi.org/10.1016/j.jsb.2005.09.004.
Suh, S. W., Jensen, K. B., Jensen, M. S., Silva, D. S., Kesslak, P. J., Danscher, G., and Frederickson, C. J. (2000) Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains, Brain Res., 852, 274-278, https://doi.org/10.1016/S0006-8993(99)02096-X.
DeBenedictis, C. A., Raab, A., Ducie, E., Howley, S., Feldmann, J., Grabrucker, A. M. (2020) Concentrations of essential trace metals in the brain of animal species – a comparative study, Brain Sci., 10, 460, https://doi.org/10.3390/brainsci10070460.
Andreini, C., Banci, L., Bertini, I., and Rosato, A. (2006) Counting the zinc-proteins encoded in the human genome, J. Proteome Res., 5, 196-201, https://doi.org/10.1021/pr050361j.
Krall, R. F., Tzounopoulos, T., and Aizenman, E. (2021) The function and regulation of zinc in the brain, Neuroscience, 457, 235-258, https://doi.org/10.1016/j.neuroscience.2021.01.010.
Prasad, A. S. (2008) Clinical, immunological, anti-inflammatory and antioxidant roles of zinc, Exp. Gerontol., 43, 370-377, https://doi.org/10.1016/j.exger.2007.10.013.
Tapiero, H., and Tew, K. D. (2003) Trace elements in human physiology and pathology: zinc and metallothioneins, Biomed. Pharmacother., 57, 399-411, https://doi.org/10.1016/S0753-3322(03)00081-7.
Cuajungco, M. P., and Fagét, K. Y. (2003) Zinc takes the center stage: its paradoxical role in Alzheimer’s disease, Brain Res. Rev., 41, 44-56, https://doi.org/10.1016/S0165-0173(02)00219-9.
Bush, A. I., Pettingell, W. H., Jr., Paradis, M. D., and Tanzi, R. E. (1994) Modulation of A beta adhesiveness and secretase site cleavage by zinc, J. Biol. Chem., 269, 12152-12158, https://doi.org/10.1016/S0021-9258(17)32694-7.
Liu, S.-T., Howlett, G., and Barrow, C. J. (1999) Histidine-13 is a crucial residue in the zinc ion-induced aggregation of the Aβ peptide of Alzheimer’s disease, Biochemistry, 38, 9373-9378, https://doi.org/10.1021/bi990205o.
Huang, X., Cuajungco, M. P., Atwood, C. S., Moir, R. D., Tanzi, R. E., and Bush, A. I. (2000) Alzheimer’s disease, β-amyloid protein and zinc, J. Nutrition, 130, 1488S-1492S, https://doi.org/10.1093/jn/130.5.1488S.
Miura, T., Suzuki, K., Kohata, N., and Takeuchi, H. (2000) Metal binding modes of Alzheimer’s amyloid beta-peptide in insoluble aggregates and soluble complexes, Biochemistry, 39, 7024-7031, https://doi.org/10.1021/bi0002479.
Yang, D. S., McLaurin, J., Qin, K., Westaway, D., and Fraser, P. E. (2000) Examining the zinc binding site of the amyloid-beta peptide, Eur. J. Biochem., 267, 6692-6698, https://doi.org/10.1046/j.1432-1327.2000.01767.x.
Kozin, S. A., Zirah, S., Rebuffat, S., Hui Bon Hoa, G., and Debey, P. (2001) Zinc binding to Alzheimer’s Aβ(1-16) peptide results in stable soluble complex, Biochem. Biophys. Res. Commun., 285, 959-964, https://doi.org/10.1006/bbrc.2001.5284.
Zirah, S., Rebuffat, S., Kozin, S. A., Debey, P., Fournier, F., Lesage, D., and Tabet, J.-C. (2003) Zinc binding properties of the amyloid fragment Aβ(1-16) studied by electrospray-ionization mass spectrometry, Int. J. Mass Spectrom., 228, 999-1016, https://doi.org/10.1016/S1387-3806(03)00221-5.
Zirah, S., Stefanescu, R., Manea, M., Tian, X., Cecal, R., Kozin, S. A., Debey, P., Rebuffat, S., and Przybylski, M. (2004) Zinc binding agonist effect on the recognition of the β-amyloid (4-10) epitope by anti-β-amyloid antibodies, Biochem. Biophys. Res. Commun., 321, 324-328, https://doi.org/10.1016/j.bbrc.2004.06.150.
Zirah, S., Kozin, S. A., Mazur, A. K., Blond, A., Cheminant, M., Segalas-Milazzo, I., Debey, P., and Rebuffat, S. (2006) Structural changes of region 1-16 of the Alzheimer disease amyloid β-peptide upon zinc binding and in vitro aging, J. Biol. Chem., 281, 2151-2161, https://doi.org/10.1074/jbc.M504454200.
Roher, A. E., Lowenson, J. D., Clarke, S., Wolkow, C., Wang, R., Cotter, R. J., Reardon, I. M., Zürcher-Neely, H. A., Heinrikson, R. L., Ball, M. J., and Greenberg, B. D. (1993) Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer’s disease, J. Biol. Chem., 268, 3072-3083, https://doi.org/10.1016/S0021-9258(18)53661-9.
Tsvetkov, P. O., Kulikova, A. A., Golovin, A. V., Tkachev, Y. V., Archakov, A. I., Kozin, S. A., and Makarov, A. A. (2010) Minimal Zn2+ binding site of amyloid-β, Biophys. J., 99, L84-L86, https://doi.org/10.1016/j.bpj.2010.09.015.
Talmard, C., Guilloreau, L., Coppel, Y., Mazarguil, H., and Faller, P. (2007) Amyloid-beta peptide forms monomeric complexes with Cu(II) and Zn(II) prior to aggregation, ChemBioChem, 8, 163-165, https://doi.org/10.1002/cbic.200600319.
Tsvetkov, P. O., Popov, I. A., Nikolaev, E. N., Archakov, A. I., Makarov, A. A., and Kozin, S. A. (2008) Isomerization of the Asp7 residue results in zinc-induced oligomerization of Alzheimer’s disease amyloid β(1-16) peptide, ChemBioChem, 9, 1564-1567, https://doi.org/10.1002/cbic.200700784.
Kozin, S. A., Mezentsev, Y. V., Kulikova, A. A., Indeykina, M. I., Golovin, A. V., Ivanov, A. S., Tsvetkov, P. O., and Makarov, A. A. (2011) Zinc-induced dimerization of the amyloid-β metal-binding domain 1-16 is mediated by residues 11-14, Mol. BioSystems, 7, 1053-1055, https://doi.org/10.1039/c0mb00334d.
Kulikova, A. A., Tsvetkov, P. O., Indeykina, M. I., Popov, I. A., Zhokhov, S. S., Golovin, A. V., Polshakov, V. I., Kozin, S. A., Nudler, E., and Makarov, A. A. (2014) Phosphorylation of Ser8 promotes zinc-induced dimerization of the amyloid-beta metal-binding domain, Mol. BioSystems, 10, 2590-2596, https://doi.org/10.1039/C4MB00332B.
Kumar, S., Rezaei-Ghaleh, N., Terwel, D., Thal, D. R., Richard, M., Hoch, M., Mc Donald, J. M., Wüllner, U., Glebov, K., Heneka, M. T., Walsh, D. M., Zweckstetter, M., and Walter, J. (2011) Extracellular phosphorylation of the amyloid β-peptide promotes formation of toxic aggregates during the pathogenesis of Alzheimer’s disease, EMBO J., 30, 2255-2265, https://doi.org/10.1038/emboj.2011.138.
Kozin, S. A., Kulikova, A. A., Istrate, A. N., Tsvetkov, P. O., Zhokhov, S. S., Mezentsev, Y. V., Kechko, O. I., Ivanov, A. S., Polshakov, V. I., and Makarov, A. A. (2015) The English (H6R) familial Alzheimer’s disease mutation facilitates zinc-induced dimerization of the amyloid-β metal-binding domain, Metallomics, 7, 422-425, https://doi.org/10.1039/C4MT00259H.
Istrate, A. N., Kozin, S. A., Zhokhov, S. S., Mantsyzov, A. B., Kechko, O. I., Pastore, A., Makarov, A. A., and Polshakov, V. I. (2016) Interplay of histidine residues of the Alzheimer’s disease Aβ peptide governs its Zn-induced oligomerization, Sci. Rep., 6, 21734, https://doi.org/10.1038/srep21734.
Gnoth, K., Piechotta, A., Kleinschmidt, M., Konrath, S., Schenk, M., Taudte, N., Ramsbeck, D., Rieckmann, V., Geissler, S., Eichentopf, R., Barendrecht, S., Hartlage-Rübsamen, M., Demuth, H.-U., Roßner, S., Cynis, H., Rahfeld, J.-U., and Schilling, S. (2020) Targeting isoaspartate-modified Aβ rescues behavioral deficits in transgenic mice with Alzheimer’s disease-like pathology, Alzheimers Res. Ther., 12, 149, https://doi.org/10.1186/s13195-020-00719-x.
Moro, M. L., Phillips, A. S., Gaimster, K., Paul, C., Mudher, A., Nicoll, J. A. R., and Boche, D. (2018) Pyroglutamate and Isoaspartate modified Amyloid-Beta in ageing and Alzheimer’s disease, Acta Neuropathol. Commun., 6, 3, https://doi.org/10.1186/s40478-017-0505-x.
Paoletti, P., Vergnano, A. M., Barbour, B., and Casado, M. (2009) Zinc at glutamatergic synapses, Neuroscience, 158, 126-136, https://doi.org/10.1016/j.neuroscience.2008.01.061.
Xie, X. M., and Smart, T. G. (1991) A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission, Nature, 349, 521-524, https://doi.org/10.1038/349521a0.
Kozin, S. A., Cheglakov, I. B., Ovsepyan, A. A., Telegin, G. B., Tsvetkov, P. O., Lisitsa, A. V., and Makarov, A. A. (2013) Peripherally applied synthetic peptide isoAsp7-Aβ(1-42) triggers cerebral β-amyloidosis, Neurotox. Res., 24, 370-376, https://doi.org/10.1007/s12640-013-9399-y.
Kulikova, A. A., Cheglakov, I. B., Kukharsky, M. S., Ovchinnikov, R. K., Kozin, S. A., and Makarov, A. A. (2016) Intracerebral injection of metal-binding domain of abeta comprising the isomerized Asp7 increases the amyloid burden in transgenic mice, Neurotox. Res., 29, 551-557, https://doi.org/10.1007/s12640-016-9603-y.
Barykin, E. P., Petrushanko, I. Y., Kozin, S. A., Telegin, G. B., Chernov, A. S., Lopina, O. D., Radko, S. P., Mitkevich, V. A., and Makarov, A. A. (2018) Phosphorylation of the amyloid-beta peptide inhibits zinc-dependent aggregation, prevents Na,K-ATPase inhibition, and reduces cerebral plaque deposition, Front. Mol. Neurosci., 11, 302, https://doi.org/10.3389/fnmol.2018.00302.
Kozin, S. A., Barykin, E. P., Telegin, G. B., Chernov, A. S., Adzhubei, A. A., Radko, S. P., Mitkevich, V. A., and Makarov, A. A. (2018) Intravenously injected amyloid-β peptide with isomerized Asp7 and phosphorylated Ser8 residues inhibits cerebral β-amyloidosis in AβPP/PS1 transgenic mice model of Alzheimer’s disease, Front. Neurosci., 12, 518, https://doi.org/10.3389/fnins.2018.00518.
Alexander, A. G., Marfil, V., and Li, C. (2014) Use of Caenorhabditis elegans as a model to study Alzheimer’s disease and other neurodegenerative diseases, Front. Genet., 5, 279, https://doi.org/10.3389/fgene.2014.00279.
Chen, X., Barclay, J. W., Burgoyne, R. D., and Morgan, A. (2015) Using C. elegans to discover therapeutic compounds for ageing-associated neurodegenerative diseases, Chem. Central J., 9, 65, https://doi.org/10.1186/s13065-015-0143-y.
Dostal, V., and Link, C. D. (2010) Assaying β-amyloid toxicity using a transgenic C. elegans model, J. Visual. Exp., 44, e2252, https://doi.org/10.3791/2252.
Ewald, C. Y., and Li, C. (2010) Understanding the molecular basis of Alzheimer’s disease using a Caenorhabditis elegans model system, Brain Struct. Funct., 214, 263-283, https://doi.org/10.1007/s00429-009-0235-3.
Earley, B. J., Mendoza, A. D., Tan, C. H., and Kornfeld, K. (2021) Zinc homeostasis and signaling in the roundworm C. elegans, Biochim. Biophys. Acta Mol. Cell Res., 1868, 118882, https://doi.org/10.1016/j.bbamcr.2020.118882.
Kumar, J., Barhydt, T., Awasthi, A., Lithgow, G. J., Killilea, D. W., and Kapahi, P. (2016) Zinc Levels modulate lifespan through multiple longevity pathways in Caenorhabditis elegans, PLoS One, 11, e0153513, https://doi.org/10.1371/journal.pone.0153513.
Shimizu, T., Watanabe, A., Ogawara, M., Mori, H., and Shirasawa, T. (2000) Isoaspartate formation and neurodegeneration in Alzheimer’s disease, Arch. Biochem. Biophys., 381, 225-234, https://doi.org/10.1006/abbi.2000.1955.
Mitkevich, V. A., Barykin, E. P., Eremina, S., Pani, B., Katkova-Zhukotskaya, O., Polshakov, V. I., Adzhubei, A. A., Kozin, S. A., Mironov, A. S., Makarov, A. A., and Nudler, E. (2022) Zn-dependent β-amyloid aggregation and its reversal by the tetrapeptide HAEE, Aging Disease, 13, 1-10, https://doi.org/10.14336/AD.2022.0827.
Tsvetkov, P. O., Cheglakov, I. B., Ovsepyan, A. A., Mediannikov, O. Y., Morozov, A. O., Telegin, G. B., and Kozin, S. A. (2015) Peripherally applied synthetic tetrapeptides HAEE and RADD slow down the development of cerebral beta-amyloidosis in abetaPP/PS1 transgenic mice, J. Alzheimers Disease, 46, 849-853, https://doi.org/10.3233/JAD-150031.
Medvedev, A. E., Buneeva, O. A., Kopylov, A. T., Mitkevich, V. A., Kozin, S. A., Zgoda, V. G., and Makarov, A. A. (2016) Chemical modifications of amyloid-beta(1-42) have a significant impact on the repertoire of brain amyloid-beta(1-42) binding proteins, Biochimie, 128-129, 55-58, https://doi.org/10.1016/j.biochi.2016.07.001.
Ershov, P. V., Mezentsev, Yu. V., Yablokov, E. O., Kaluzhskiy, L. A., Ivanov, A. S., Gnuchev, N. V., Mit'kevich, V. A., Makarov, A. A., and Kozin, S. A. (2020) Direct molecular fishing of zinc-dependent protein partners of amyloid beta 1-16 with Taiwan mutation (D7H) and phosphorylated SER8, Mol. Biol., 54, 904-910, https://doi.org/10.1134/S0026893320060035.
Ivanov, A. S., Ershov, P. V., Mol’nar, A. A., Mezentsev, Yu. V., Kaluzhskiy, L. A., Yablokov, E. O., Florinskaya, A. V., Gnedenko, O. V., Medvedev, A. E., Kozin, S. A., Mit’kevich, V. A., Makarov, A. A., Gilep, A. A., Lushchik, A. Ya., Gaydukevich, I. V., and Usanov, S. A. (2016) Direct molecular fishing in investigating molecular partners of protein–protein and protein–peptide interactions, Russ. J. Bioorg. Chem., 42, 14-21, https://doi.org/10.1134/S1068162016010052.
Deigin, V. I., Poluektova, E. A., Beniashvili, A. G., Kozin, S. A., and Poluektov, Y. M. (2022) Development of peptide biopharmaceuticals in Russia, Pharmaceutics, 14, 716, https://doi.org/10.3390/pharmaceutics14040716.
Kozin, S. A., Barykin, E. P., Mit’kevich, V. A., Makarov, A. A. (2018) Anti-amyloid therapy of Alzheimer’s disease: current state and prospects, Biochemistry (Moscow), 83, 1057-1067, https://doi.org/10.1134/S0006297918090079.
Kozin, S. A., and Makarov, A. A. (2019) The convergence of Alzheimer’s disease pathogenesis concepts, Mol. Biol., 53, 896-903, https://doi.org/10.1134/S0026893319060104.
Kozin, S. A., Pol’shakov, V. I., Mezentsev, Yu. V., Ivanov, A. S., Zhokhov, S. S., Yurinskaya, M. M., Vinokurov, M. G., Makarov, A. A., and Mit'kevich, V. A. (2018) Enalaprilat inhibits zinc-dependent oligomerization of metal-binding domain of amyloid-beta isoforms and protects human neuroblastoma cells from toxic action of these isoforms, Mol. Biol., 52, 590-597, https://doi.org/10.1134/S0026893318040106.
Funding
The study was financially supported by the Russian Science Foundation (grant no. 19-74-30007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflicts of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Translated from Uspekhi Biologicheskoi Khimii, 2023, Vol. 63, pp. 149-174.
Rights and permissions
About this article
Cite this article
Kozin, S.A. Role of Interaction between Zinc and Amyloid Beta in Pathogenesis of Alzheimer’s Disease. Biochemistry Moscow 88 (Suppl 1), S75–S87 (2023). https://doi.org/10.1134/S0006297923140055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923140055