Abstract
Currently, nucleic acid therapeutics are actively developed for the treatment and prophylactic of metabolic disorders and oncological, inflammatory, and infectious diseases. A growing number of approved nucleic acid-based drugs evidences a high potential of gene therapy in medicine. Therapeutic nucleic acids act in the cytoplasm, which makes the plasma membrane the main barrier for the penetration of nucleic acid-based drugs into the cell and requires development of special vehicles for their intracellular delivery. The optimal carrier should not only facilitate internalization of nucleic acids, but also exhibit no toxic effects, ensure stabilization of the cargo molecules, and be suitable for a large-scale and low-cost production. Cell-penetrating peptides (CPPs), which match all these requirements, were found to be efficient and low-toxic carriers of nucleic acids. CPPs are typically basic peptides with a positive charge at physiological pH that can form nanostructures with negatively charged nucleic acids. The prospects of CPPs as vehicles for the delivery of therapeutic nucleic acids have been demonstrated in numerous preclinical studies. Some CPP-based drugs had successfully passed clinical trials and were implemented into medical practice. In this review, we described different types of therapeutic nucleic acids and summarized the data on the use of CPPs for their intracellular delivery, as well as discussed, the mechanisms of CPP uptake by the cells, as understanding of these mechanisms can significantly accelerate the development of new gene therapy approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Over the past two decades, the number of new drugs using nucleic acids as pharmaceutical agents has significantly increased – e.g., RNA and DNA vaccines that have found a broad application in medical practice [1]. Many currently developed drugs contain antisense oligonucleotides (ASOs), aptamers, small interfering RNAs (siRNAs), and other types of oligonucleotides [2].
Despite an enormous therapeutic potential, the use of nucleic acids poses a number of problems; for example, nucleic acids are degraded by body nucleases and can be eliminated by the phagocytic system and liver enzymes. However, the main challenge remains an efficient delivery of these therapeutic molecules to the site of action [3], which has been partially solved by using various viral and non-viral delivery vectors. Although lentiviral and adenoviral vectors demonstrate a very high efficiency, they also have a number of disadvantages [4, 5]. The most commonly used non-viral vectors are nanoparticles (liposomes, polymer nanoparticles) and peptides [6].
The use of peptide carriers for the delivery of therapeutic molecules to target cells has been actively developed in the last few decades. At present, cell-penetrating peptides (CPPs) are tested for the treatment of oncological diseases (breast, colorectal, and lung cancers, etc.) as therapeutic agents with their own antitumor (oncolytic) activity, as well as vehicles for the targeted delivery of anticancer drugs [7-9]. CPPs can deliver therapeutic molecules not only to the target cells, but even to cell organelles. For example, TAT-based peptides are able to penetrate into the mitochondria and deliver the antioxidant protein hMT1A, which reduces formation of reactive oxygen species and thereby prevents manifestations of the Parkinson’s disease in experimental models [10, 11]. CPPs have been also studied for the suppression of inflammatory processes [9]. Thus, CPPs have been developed against the STAT3 protein, the inhibition of which significantly reduces inflammation in psoriasis. CPP-based anti-inflammatory drugs are often administered transdermally due to their ability to penetrate through the cornified layer of the skin [11, 12]. A number of studies describe the use of CPPs in ophthalmology, as they can be applied in a form of eye drops. TAT peptide-based preparation XG-102 has successfully completed Phase 3 clinical trials for reduction of eye inflammation after cataract surgery (NCT02508337). Other peptide-based drugs (KAI, POD, TAT-aFGF-His, and TAT-µCL) are under development [13]. The use of CPPs for the delivery of oral insulin as an alternative to subcutaneous injection is of particular interest, because the bioavailability of orally administered insulin without a carrier is low [11, 14]. Another area of CPP application is the treatment and prevention of viral infections. To date, CPP-based antiviral drugs and vaccines [15] have been created against hepatitis C virus, rhinovirus, coxsackievirus, respiratory syncytial virus, Ebola virus, influenza type A virus, HIV-1, papilloma virus, herpes virus, SARS-CoV-2, and others [16].
Peptide vehicles for the delivery of therapeutic nucleic acids has been actively created in recent decades. The advantages of CPPs in comparison to other vectors include their biodegradability, biocompatibility, and a relative ease of production, standardization, and storage. Unlike viral vectors, these peptide vehicles are not immunogenic and do not cause mutations [15]. In this review, we summarized recent developments in the design and application of peptide-based vehicles for the delivery of therapeutic nucleic acids.
THERAPEUTIC NUCLEIC ACIDS
Due to their high specificity, functional diversity, and low toxicity, therapeutic nucleic acids have attracted a lot of interest as potential drugs. Most of these compounds [ASOs, ribozymes, DNAzymes, external guide sequences (EGSs), siRNAs, etc.] selectively suppress expression of target genes through various mechanisms (Table 1). At least 15 nucleic acid-based drugs have been approved for medical use [2]; seven of them utilize non-viral delivery vehicles [2, 17].
ASOs are typically single-stranded synthetic DNA oligonucleotides 15-25 nt long. They hybridize with the target mRNA according to the complementarity principle, creating steric hindrances for the protein translation. Some ASOs are designed in such a way that their interaction with the target mRNA activates RNase H, which cleaves the target mRNA, thus increasing the efficacy of gene suppression. Several generations of ASOs have been developed.
First-generation ASOs are synthesized from modified nucleotides with the formation of phosphorothioate oligonucleotides resistant to the cleavage by nucleases. They recruit RNase H for the degradation of the target mRNA. The disadvantage of first-generation ASOs is that they can trigger unwanted immune response or nonspecifically interact with cellular proteins, which might have a toxic effect on the cells
Second-generation ASOs, or the so-called 2′-O-(2-methoxyethyl)-oligoribonucleotides (2′-MOE oligonucleotides), are synthesized from modified nucleotides containing an alkyl modification at the 2′-position of ribose. These ASOs are resistant to nucleases and interact with the target mRNAs with an increased affinity. However, second-generation ASOs are less efficient compared to the first-generation ASOs, since they do not recruit RNase H, so that suppression of the gene expression occurs due to the steric blockade of translation enzymes.
Third-generation ASO consists of nucleotides with other modifications and include peptide nucleic acids (PNAs), 2′-deoxy-2′-fluoro-β-D-arabinonucleic acids (FANAs), locked nucleic acids (LNAs), cyclohexene nucleic acids (CeNAs), etc. [3] that are resistant to the degradation by nucleases. Two ASO-based drugs have been approved for medical use – fomivirsen (first-generation ASO-based drug) for the treatment of cytomegalovirus retinitis and mipomersen (second-generation ASO-based drug) for the treatment of hypercholesterolemia. Over 50 third-generation ASOs are currently tested in clinical trials [18].
Aptamers are single-stranded synthetic DNA or RNA molecules 56-120 nt long that are similar in structure to ASOs. Aptamers act as proteins traps; they bind to the target protein and interfere with its functioning. Unlike ASOs, aptamers exert their effect not through the complementary interactions of nucleotide bases, but by binding to the target protein (similar to antibodies) and, therefore, represent an attractive alternative to monoclonal antibodies. Aptamers are low-toxic, non-immunogenic, and relatively easy to obtain. They penetrate well into the tissues and can be administered either intravenously or subcutaneously [3].
Ribozymes are catalytically active single-stranded RNA molecules that selectively cleave target mRNAs. Due to their tertiary structure with two loops, ribozymes bind to the target mRNA and form a catalytic core that cleaves the mRNA molecule. However, ribozymes are not very resistant to RNases, and therefore require stabilization, which increases the cost of their synthesis.
DNAzymes are catalytically active single-stranded DNA molecules. Like ribozymes, they form their own catalytic site and cleave mRNA molecules without involving nucleases. DNAzymes are more stable and less expensive to produce than ribozymes, but at the same time, they are larger and more difficult to synthesize than siRNAs.
EGSs (external guide sequences) are synthetic single-stranded RNA oligonucleotides 15-20 nt in length. In the cytoplasm, EGSs bind to the target mRNA according to the complementarity, after which the mRNA is cleaved in the duplex region by RNase P. Due to the enzyme activation, EGS efficiently suppress the target gene; however, they are susceptible to the degradation by RNases [19].
DNA traps are double-stranded DNA oligonucleotides that mimic gene promoter regions. Because of this similarity, they bind to transcription factors, thereby competing with natural genes and suppressing their activity [20].
U1 adapters are synthetic oligonucleotides 30-50 nt in length composed of ribonucleotides, deoxyribonucleotides, or modified nucleotides. U1 adapter consists of the 5′-end targeting sequence designed to be complementary to the 3′-terminal exon of the target mRNA, which provides its recognition by the U1 adapter, and conserved U1 domain capable of hybridizing with the small nuclear RNA (snRNA), a component of the ribonucleoprotein complex with the nuclease activity. Gene suppression by U1 adapters occurs in the nucleus (and not in the cytoplasm), where U1 adapter specifically interacts with the precursor mRNA (pre-mRNA) through the targeting sequence and with the ribonucleoprotein complex through the conserved U1 domain, resulting in the pre-mRNA cleavage in the polyadenylation region. Pre-mRNA lacking the poly-A tail, which protects it from the action of RNases, is quickly degraded, leading to the reduced expression of the target gene [21].
siRNAs are double-stranded RNA molecules 21-25 bp in length that can specifically suppress gene expression via the RNA interference (RNAi) mechanism [22] (Fig. 1), which includes the initiation and effector stages [23]. At the initiation stage, cytoplasmic nuclease Dicer cleaves long double-stranded RNA molecules (e.g., formed as a result of viral infection) into short duplexes (siRNAs). At the effector stage, siRNA combines with protein factors, forming the multisubunit RNA-induced silencing complex (RISC), in which one of the two siRNA strands, the so-called guide (antisense) strand, associates with the Argonaute (AGO) protein and ensures the specificity of the RISC interaction with the target mRNA, while the other (passenger) strand is degraded. After binding to the target mRNA, RISC introduces initial breaks, producing mRNA fragments that are further degraded by cellular exonucleases. Introduction of artificial siRNAs of a required size (21 bp) into cells allows to avoid the processing by Dicer, so that they can be immediately included in the RISC complex and specifically suppress expression of the target genes. Unlike single-stranded ribozymes, EGSs, and U1 adapters, double-stranded siRNAs are more resistant to RNases. In addition, their stability and efficiency can be increased by nucleotide modification [24]. To date, more than 30 clinical trials of siRNA-based drugs have been initiated. In 2022, six siRNA-based drugs against hepatitis B virus (HBV) were undergoing clinical trials: ARC-520, ARB-1467, AB-729, RG-6346, VIR-2218, and JNJ-3989 [25]. The first registered siRNA-based drug was Inclesiran (developed by Alnylam Pharmaceuticals, licensed by Novartis) for the treatment of patients with atherosclerotic cardiovascular disease and hypercholesterolemia [26]. Later, the FDA (Food and Drug Administration) approved three more drugs based on siRNAs (Patisiran, Givosiran, and Lumasiran) [27]. The drug MIR 19 was registered in the Russian Federation for the treatment of COVID-19 [17, 28].
RNAi mechanism. At the initiation stage (I), dsRNA is cleaved by the enzyme Dicer to form siRNAs. At the effector stage (II), siRNA binds to RISC; the helicase of the complex unwinds the siRNA molecule; the antisense (guide) strand associates with the AGO protein and remains in the complex, while the sense (passenger) strand is displaced from the complex and degraded. The guide strand directs the complex to the target mRNA, resulting in its cleavage.
mRNA-based preparations. The main class of drugs containing mRNA molecules are vaccines [29]. Although this approach allows simultaneous coexpression of several target proteins from the same mRNA molecule, mRNA preparations are often low-stabile and immunogenic (since mRNAs often contain immunostimulating sequences). Also, unlike relatively small siRNAs and ASOs, the delivery of large mRNA molecules is more problematic [3]. Nevertheless, more than 60 mRNA-based preparations have been created, including vaccines against COVID-19 from Pfizer-BioNTech and Moderna [3, 30].
DNA-based preparations are plasmids carrying required genetic material. They are directly introduced into the body, where they express the target protein (antigen) [3]. Although DNA vaccines encoding protein antigens have been actively developed, their efficiency turned out to be low, which has prompted many scientific groups to work on the increase of vaccine immunogenicity and optimization of its composition. Currently, the most promising approach for the DNA delivery in gene therapy is the use of viral vectors. This approach was implemented in the development of such drugs as the herpes simplex virus-based Imlygic® (talimogene laherparepvec) for the treatment of melanomas, adeno-associated virus-based Luxturna® (voretigene neparvovec) for the treatment of retinal dystrophy, adeno-associated virus-based Zolgensma® (onasemnogene abeparvovec) for the therapy of spinal muscular atrophy, and others [31].
Of the technologies described above, the most promising technology in terms of efficiency and economic feasibility is RNAi. The attempts to introduce it into medical practice have started immediately after discovery of this phenomenon and elucidation of its molecular mechanism. However, implementation of RNAi technology is hindered by a low stability of siRNA molecules in blood serum and lack of highly efficient and safe agents for their targeted delivery to the cytoplasm of target cells. Currently, cationic liposomes, peptides, polymers, and modified RNase-resistant nucleic acids are studied as potential non-viral delivery vehicles, which might soon provide new possibilities for the application of therapeutic nucleic acids.
NON-VIRAL SYSTEMS FOR THE NUCLEIC ACID DELIVERY
Non-viral vehicles include liposomes, exosomes, nanoparticles of various nature, cationic polymers, peptides, etc. [4].
Liposomes are one of the most commonly used vehicles for the nucleic acid delivery. Typically, they consist of cationic and/or amphiphilic lipids and cholesterol. Their popularity is explained by a relatively easy formation of complexes with nucleic acids (often due to electrostatic interaction), high efficiency of delivery to target cells, as well as the fact that the liposome surface can be modified with various ligands to increase its tropism to the target cells and tissues. However, the disadvantages of liposomes include their low stability (because of sedimentation), high toxicity, and the need for additional quality control during production. Currently, at least three liposome-based RNA drugs have been registered, including Patisiran from Alnylam, BNT162b2 from Pfizer, and mRNA-1273 from Moderna [6].
Exosomes are natural nanoparticles (vesicles) encapsulated in a lipid bilayer, that are formed by budding from eukaryotic cells. It is believed that exosomes are involved in the cell-to-cell communication by transporting macromolecules (nucleic acids, proteins, and lipids), which makes them of interest as potential vehicles for delivery of therapeutic oligonucleotides. They are less toxic than liposomes and are able to pass through biological barriers (e.g., the blood-brain barrier). However, exosomes bind transported nucleic acids less efficiently than liposomes. At presents, several exosome-based drugs (from Codiak Biosciences and Evox Therapeutics) are tested in preclinical studies [32].
Nanoparticles of various kinds are also used for the delivery of nucleic acids. For example, hybrid nanoparticles consisting of different types of molecules (lipids, polymers, and peptides) can combine the advantages of each component [4]. Vectors based on gold nanoparticles [4], silicon dioxide, and other materials [6] have also been described.
Polymer-based vectors present a number of advantages over liposomes. In particular, they are less immunogenic and can be easily modified, which allows to change their physicochemical properties (charge, stability and molecular weight) and thus regulate their solubility, tissue affinity, and pharmacokinetics. Some of the first polymers to be used for the delivery of nucleic acids were polyethyleneimine and its derivatives, but the widespread use of these compounds has been limited by their toxicity [33, 34]. Another polymer, poly(D,L-lactide-co-glycolide) (PLGA), is non-toxic and has been approved for medical use. However, due to the lack of positive charge, it does not interact electrostatically with nucleic acids. Nevertheless, work is underway to create its derivatives adapted for the delivery of therapeutic nucleic acids [34]. Dendritic (branched) polymers, e.g., poly(amidoamine) (PAMAM) dendrimers, are characterized by a large number of functional groups that can be modified to achieve necessary biological properties. However, such polymers have a number of disadvantages, such as short half-life and toxicity [4].
In order to achieve a therapeutic effect, delivery systems should be non-toxic, stable in biological media, and able to bind nucleic acids and penetrate into the target cells. Other important aspects that can affect introduction of such delivery systems into broad medical practice are relative ease of synthesis, quality control, and possibility of production scaling up. Most of these requirements are met by the CPP-based vehicles.
STRUCTURE AND CLASSIFICATION OF TRANSPORT PEPTIDES
CPPs usually consist of 5-30 amino acid (aa) residues, a significant number of which are basic amino acids (arginine and lysine) to provide a positive charge to the peptide molecule in aqueous solutions at physiological pH values. CPPs often have the amphipathic nature, i.e., one part of the peptide is hydrophobic and the other is hydrophilic. CPPs not only penetrate into the cell, but can also transport various therapeutic agents (for example, nucleic acids [24]) to the cytoplasm [35, 36].
The history of CPP research has begun with the discovery of human immunodeficiency virus TAT protein capable of penetrating and transporting various compounds into mammalian cells. Currently, the CPPsite 2.0 database (http://crdd.osdd.net/raghava/cppsite/) describes more than 1700 unique peptides with the transport activity. The properties and characteristics of CPPs have been described in recent reviews [37-39].
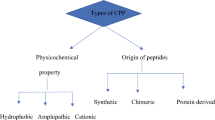
There are several classifications of CPPs. According to the one based on peptide size and physicochemical properties, CPPs are divided into primary amphipathic, secondary amphipathic, and non-amphipathic.
Primary amphipathic peptides usually contain more than 20 aa, both hydrophilic and hydrophobic, in their structure. This group includes MPG, penetratin, CADY, pVec, and other peptides [40].
Secondary amphipathic peptides are usually less than 20 aa in size and have an α-helical or β-sheet secondary structure. Secondary α-helical CPPs typically have a hydrophobic region at one end, while the other end can be hydrophilic (cationic or anionic).
Non-amphipathic peptides (e.g., HIV-TAT) are relatively short and have a high content of positively charged amino acids (arginine or lysine). Research shows that an efficient cellular uptake of such peptide requires at least eight positive charges [40].
A separate group is cationic dendrimers characterized by a branched structure and high positive charge density due to the high content of lysine or arginine residues. These peptides have significant advantages over linear peptides, as they are more resistant to proteolysis due to the non-natural ε-amide bonds, while the presence of a large number of charged terminal groups ensures efficient binding of nucleic acids. Dendrimeric CPPs also have much lower cytotoxicity than linear CPPs, which is of great importance for their use in medicine.
Therefore, due to the diversity of structures and physicochemical properties, peptide carriers represent a promising class of compounds for the intracellular delivery of therapeutic nucleic acids. Moreover, this structural diversity underlies the diversity of molecular mechanisms used by peptides to penetrate into the target cells.
MOLECULAR MECHANISMS OF CPP INTERNALIZATION
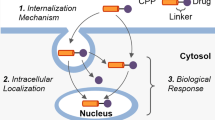
Mechanisms of CPP internalization have been studied by many different methods that can be found in the review by Holm et al. [41]. In particular, studying the ability (or inability) of a peptide to penetrate into eukaryotic cells in the presence of endocytosis inhibitors helps to elucidate the mechanism of penetration. CPPs can also be conjugated with fluorescent labels, which allows to use confocal microscopy for identification of cell compartments in which CPPs accumulate, as well as to evaluate the kinetics of this accumulation in the cells, which ultimately helps in the identification of molecular mechanisms of peptide internalization.
According to modern concepts [37, 40], there are several mechanisms of CPP penetration into the cells that can be broadly divided into the energy-independent direct penetration and energy-dependent pathways (various types of endocytosis).
Energy-independent direct penetration of CPPs includes two stages: (i) CPP interaction with the cell membrane; (ii) penetration into the cytoplasm through the formation of pores, adaptive translocation, or formation of inverted micelles (Fig. 2a). This mechanism is typical for CPPs rich in arginine and lysine [42], the presence of which provides a positive charge allowing the peptide to electrostatically interact with negatively charged proteoglycans or glycosaminoglycans of the cell wall and thereby accumulate on the membrane surface. When the maximum concentration of the peptide is reached, the structure of lipids in the membrane bilayer is destabilized, so that the peptide penetrates into the cytoplasm through the formed pores (Fig. 2b). The pores in the membrane are formed for a relatively short period of time, which allows the peptide to enter the cell without causing cell lysis and death. However, the formation of pores in the cell membrane at high CPP concentrations can be detrimental to the cell.
Mechanisms of energy-independent penetration of CPPs through the cell membrane. a) Electrostatic interaction of positively charged CPPs with the negatively charged cell membrane, followed by peptide penetration via (1) pore formation, (2) adaptive translocation (“squeezing” through the membrane phospholipids), and (3) formation of inverted micelles by membrane invagination caused by positively charged peptides and their capture from the extracellular environment. b) Molecular models of pore formation. The barrel-stave model (1) assumes formation of peptides clusters with the channels in their centers, through which CPPs penetrate to the cytosol. The toroidal model (2) is based on the interaction between the positively charged peptide side chains and phosphate groups of the cell membrane lipids, leading to the inward bending of the lipid monolayer with the formation of a hydrophilic gap in the membrane and CPP penetration into the cell. The carpet-like model (3) suggests accumulation of peptides on the cell surface with the formation of a carpet-like layer, followed by rearrangement of the negative charges of membrane phospholipids as a result of their interaction with the cationic groups of CPP, which causes a decrease in the local surface tension and promotes CPP intercalation into the cell membrane. Molecular electroporation (4) of CPP into the cell is possible due to the binding of the highly charged peptide to the membrane, formation of a local electric field, and rupture of the cell membrane. In the molecular gap model (5), the peptides are oriented perpendicular to the membrane and aggregate with each other via their lateral surfaces, forming a “ribbon” that penetrates into the cell through a gap in the cell membrane phospholipids. The sinking raft model (6) describes a process in which peptides form aggregates that diffuse across the membrane parallel to the phospholipid bilayer.
There are several molecular models describing the CPP-induced pore formation in the cell membrane.
The barrel-stave model (Fig. 2b) is characteristic of amphipathic α-helical peptides. After interacting with the cell membrane, the peptides form bundles that have channels in their center. The pores are formed by the inwardly facing hydrophilic surfaces and interaction of outwardly facing hydrophobic residues with the lipid membrane.
The toroidal model describes pore formation by the peptides capable of forming α-helices upon contact with the cell membrane. According to this model, the interaction between the positively charged side chains of the peptide amino acid residues and phosphate groups of the cell membrane lipids leads to the peptide accumulation on the membrane outer leaflet. The peptides then cause the lipid monolayer to bend inward, creating a hydrophilic gap in the membrane that houses the phospholipid heads and peptides.
In the carpet-like model, positively charged segments of the peptides accumulate on the surface of the cell membrane and form a carpet-like layer that causes distortion of the membrane as a result of interaction of negatively charged lipids of the outer membrane leaflet and cationic groups of CPPs. This leads to the rearrangement of the negative charges of phospholipids and thinning of the membrane. Aggregation of CPPs on the membrane surface causes a decrease in the local surface tension and promotes CPP intercalation into the cell membrane. After peptide internalization into the cytoplasm, the membrane is restored [43].
Molecular electroporation is another proposed mechanism of pore formation, in which the membrane bilayer is locally disrupted by the transmembrane electric field generated by the binding of charged CPP to the membrane (Fig. 2b).
In the leaky slit model, the peptides are oriented perpendicular to the membrane and aggregate with each other via their lateral surfaces, forming a so-called ribbon. Then the other side of the membrane bends onto itself, forming a gap [40] (Fig. 2b).
The alternative sinking raft model describes the internalization process as a formation of peptides aggregates that can diffuse through the membrane (Fig. 2b).
During adaptive translocation, positively charged CPPs form transient ionic complexes with negatively charged membrane components (Fig. 2a). The complexes have a weak positive charge (since the charge of CPP is partially neutralized), which allows the peptide to adaptively diffuse through the cell membrane, the diffusion being driven by the membrane potential [44].
Formation of inverted micelles. Direct penetration via formation of inverted micelles was originally described for penetratin (Fig. 2a). The first step in the internalization process is electrostatic interaction between the peptide and cell membrane, which affects supramolecular organization of membrane lipids and can lead to changes in the membrane curvature. Invagination of the cell membrane can result in the formation of inverted micelles that capture the peptide from the extracellular medium. The hydrophilic environment inside the inverted micelle allows peptide accumulation. Inside the cell, the micelle is destabilized and the peptides are released to the cytoplasm [40].
Energy-dependent penetration of CPPs. The energy-dependent mechanisms of peptide penetration into cells include various types of endocytosis (Fig. 3). Endocytosis is a process of transporting substances into a cell that involves various organelles (membrane, microtubules, etc.) and is generally divided into two categories: phagocytosis and pinocytosis. Phagocytosis is used by specialized cells (macrophages, dendritic cells, etc.) to capture large macromolecules and particles from the extracellular environment, while pinocytosis involves the uptake of fluids and occurs in all cells. Pinocytosis includes macropinocytosis, clathrin-mediated endocytosis, caveolin-mediated endocytosis, and clathrin/caveolin-independent endocytosis with the participation of cell receptors, such as neuropilin 1 and heparan sulfate proteoglycans [40, 45]. All these types of endocytosis require energy, usually provided by ATP hydrolysis.
Energy-dependent mechanisms of CPP penetration through the cell membrane. During macropinocytosis, protrusions formed by the cell membrane collapse onto and fuse with the plasma membrane with the generation of macropinosomes, in which encapsulated CPP interacts with proteoglycans on the membrane surface (1). During clathrin-dependent endocytosis, polymerization of clathrin and actin cytoskeleton leads to the membrane invagination and formation of CPP-containing vesicles, the separation of which from the membrane is promoted by dynamin. The vesicles are released to the cell cytoplasm, followed by clathrin depolymerization (2). Caveolin-dependent endocytosis is in many ways similar to clathrin-dependent endocytosis, but the process of vesicle formation occurs with the involvement of the Cav-1, -2, and -3 proteins and cavins (3).
Macropinocytosis is a receptor-independent lipid raft-dependent form of membrane transport that requires the involvement of the actin cytoskeleton (Fig. 3). It is typically induced in response to cell stimulation by growth factors (CSF-1, EGF, etc.). During macropinocytosis, membrane protrusions do not “envelop” the particles (as in the case of phagocytosis), but collapse onto and fuse with the plasma membrane to form large endocytic vesicles called macropinosomes. In macropinocytosis, CPP interacts with proteoglycans on the membrane surface; the membrane reorganizes (after recruitment of Rac and actin) with the generation of macropinosomes in which CPP is encapsulated. It was shown that this mechanism is typical for the penetration of arginine-rich peptides (R8, R9, R12) [40].
Clathrin-dependent endocytosis is the best-characterized endocytosis mechanism (Fig. 3). Endosome generation occurs in the so-called hotspots of vesicle formation, that are usually the cell membrane zones enriched in phosphatidylinositol 4,5-bisphosphate (PIP2). PIP2 interacts with adapter proteins, the most important of which is the adapter protein 2 (AP2) complex. AP2 binds to PIP2 and recruits scaffold proteins to the plasma membrane, which initiates clathrin assembly into icosahedral structures on the inner membrane surface [44]. Accumulation of CPPs in the hotspots is followed by the membrane invagination. It is believed that polymerization of the actin cytoskeleton and clathrin promotes membrane invagination and formation of vesicles 100-150 nm in diameter [46]. The release of these vesicles from the donor membrane is promoted by the protein dynamin. Dynamin assembles into tight oligomers and constrict the membrane at the top of the nascent vesicle into a thin, hourglass-shaped bridge and then completely separates the vesicle from the membrane The vesicle is released into the cell cytoplasm, where clathrin depolymerizes, resulting in the formation of the so-called early endosome, which matures to the late endosome characterized by a low pH value. Late endosomes transport their content to the lysosomes, which contain various degradation enzymes and have even lower pH. Because of the degradation of transported substances in the lysosomes, clathrin-dependent endocytosis is less preferred for the delivery of therapeutic nucleic acids. To increase the efficiency of transport, auxiliary compounds are used that initiate early release of transported cargo from the endosome into the cell cytoplasm (see below). It was experimentally shown that many CPPs, including TAT [47], MPGα, R8 [48], etc., use this mechanism to penetrate into cells.
Caveolin-dependent endocytosis is similar to clathrin-dependent endocytosis, but has a number of distinctive features (Fig. 3). Caveolae are flask-shaped invaginations of the cell membrane, approximately 50-100 nm in diameter, that are present in many types of cells. Caveolae form in the membrane areas that are rich in cholesterol and sphingolipids and might contain molecular receptors. Some authors call these hydrophobic membrane areas lipid rafts. The main molecular components of caveolae are Cav-1, -2, and -3 proteins; the first two are coexpressed and present in a wide range of cells. Cav-1 gives the shape to caveolae. It is an integral membrane protein with hydrophobic amino acids inserted into the membrane bilayer. Another group of proteins required for caveolin-mediated endocytosis is cavins; they are located on the inner surface of the cell membrane facing the cytosol and are necessary for the interaction with the cell cytoskeleton. It has been shown that cavin-1 binding to caveolins, cholesterol, and phosphatidylserine stabilizes membrane curvature and maintains the flask-like shape of caveolae. Other important molecular components of caveolae are the actin cytoskeleton, cholesterol, and dynamin, which interacts directly with Cav-1 at the base of the caveola and ensures its budding with subsequent formation of the intracellular vesicle [40]. Internalization of caveolae leads to the formation of caveosomes, vesicles with heterogeneous morphology and neutral pH, which distinguishes them from late endosomes with low pH. Apparently, the cargo transported by caveosomes does not undergo degradation (as in lysosomes during clathrin-mediated endocytosis). However, it is still unknown how the cargo is released to the cytoplasm [40]. This mechanism is used for the cell penetration by the TAT peptide, proline-rich peptides [49], TP10 [50], etc.
Clathrin- and caveolin-independent endocytosis. Another pathway for the energy-dependent entry of CPPs into cells is clathrin- and caveolin-independent endocytosis that occurs with the involvement of lipid rafts (cell membrane areas rich in sterols and sphingolipids 40-50 nm in diameter). Lipid rafts can contain not only clathrin and caveolin, but also other macromolecules (e.g., receptor proteins) that recognize transported substances in the extracellular space and promote their uptake. A number of CPPs have been shown to enter cells using this mechanism, for example, azurin and its fragments p18 and p28 [51], transportan, transportan-10 [50], and LMWP in a complex with siRNA molecules [52]. Both transportan and transportan-10 had been previously shown to enter cells via caveolin-dependent endocytosis, suggesting that peptides can simultaneously engage several entry pathways.
The mechanism of CPP penetration depends on the physicochemical properties of the peptide and transported molecule, their concentration, and structural features of the plasma membrane. For example, under physiological conditions, peptides at low concentrations enter cells via endocytosis, while at high concentrations, CPPs directly interact with the cell membrane and use energy-independent mechanisms to enter the cells [40].
CPP endosome escape. After entering the cell via endocytosis, CPPs (and therapeutic nucleic acids they transport) accumulate in the endosomes, which mature and turn into lysosomes, leading to the degradation of the endosomal contents by hydrolases. Therefore, timely release of CPPs from the endosome is a necessary condition for the efficient delivery of the cargo molecules to the cell [39].
Destruction of endosomal membrane. The release of CPP from the endosome can be achieved using different molecular mechanisms. One of them is destruction of the endosomal membrane by CPPs with the α-helical structure [44]. Positively charged CPPs interact with negatively charged membrane phospholipids, which leads to the formation of pores and release of the endosomal content to the cytosol [53].
Formation of ion pairs. Another mechanism for the escape from the endosomes is formation of ion pairs between CPPs and negatively charged membrane lipids, after which the peptides diffuse through the membrane. This mechanism is typical for arginine-rich peptides.
Fusogenic lipids. The endosomal release of CPPs can be induced by fusogenic lipids (e.g., DOPE). At low pH values, fusogenic lipids fuse with the endosomal membrane, which ultimately destabilizes it and leads to the release of endosomal content to the cytosol. It was shown that addition of DOPE to the CPP–nucleic acid complex significantly increased the efficiency of complex penetration into the cytosol [33]. Endosomal escape can also be achieved through the use of pH-sensitive polymers and pH-sensitive liposomes [54].
The “proton sponge” mechanism describes the release of polyplexes containing polyethyleneimine and implies the presence of proton-binding molecules in the endosome (Fig. 4). This strategy was used for histidine-containing CPPs. The imidazole group of histidine gets protonated, and since endosomes contain proton pumps, additional protons are “pumped” into the endosome, followed by chloride anions. An increase in the osmotic pressure results in the rupture of endosome membrane and release of its content to the cytosol. Endosomal escape can be activated by endosomolytic agents, such as chloroquine (weak base). Chloroquine binds protons inside the endosome, thereby preventing acidification of the endosomal content, while at high concentrations, chloroquine causes swelling of the endosome and its rupture. Another way to employ the proton sponge effect to release CPPs from the endosomes is to use proton-binding polymers, such as polyethyleneimine. These polymers can be covalently attached to CPPs (or form complexes with CPPs), which slightly reduces their ability to internalize, but facilitates endosomal escape, resulting in more efficient peptide delivery [39].
The “umbrella” mechanism describes the release of large polymeric compounds from the endosome (Fig. 4). According to this hypothesis, the carrier polymer forms complexes with therapeutic nucleic acids due to electrostatic interactions, resulting in their compaction (complex condensation). Acidification of the endosomal content to pH 5-6 causes protonation of amino groups of the carrier polymer, resulting in its decondensation and expansion due to electrostatic repulsion of similarly charged amino groups. These processes lead to the increase in the endosome volume and subsequent rupture of its membrane with the release of endosomal content to the cytosol. This mechanism is characteristic of large polymer carriers and some CPPs (for example, dendrimers).
Penetration of CPPs into the nucleus. In some cases, therapeutic nucleic acids should be delivered into the cell nucleus, which requires their transportation through two barriers – the cellular and nuclear membranes. Some CPPs (TAT, polyarginine, etc.) are able to penetrate into the cell nucleus [54]. To increase nuclear tropism, CPPs can modified by conjugation with certain peptide sequences (usually containing lysine, arginine, and proline residues) recognized by nuclear transport proteins (importin-α and importin-β) [55] to promote nuclear localization of CPPs.
The nuclear pore complex (NPC) is a cylindrical structure with an internal diameter of about 30 nm and length of 50 nm, which ensures the transport of substances into the nucleus. Small molecules (water, sugars, and ions) pass through the CNP passively. However, large molecules (mRNAs, proteins, peptides, etc.) have to be transported through the CNP in an energy-dependent manner with the help of nuclear localization sequences (NLSs) [54]. NLSs are short sequences of 5-12 aa found in the nuclear proteins of eukaryotes and viruses. That can be conjugated with CPPs to target these peptides to the nucleus. The most common NLSs are peptides from SV40 large T antigen and Xenopus nucleoplasmin [54], 29-mer peptide from the cytomegalovirus pUL53 protein [56], and others [57].
The diversity of structural and physicochemical properties of CPPs, as well as a relative ease of their modification, allow the use of these peptides as vehicles for the delivery of therapeutic nucleic acids to the nucleus.
Factors affecting CPP internalization. It has been shown in many experiments that the CPP uptake pathway depends on the applied concentration. Thus, endocytosis usually occurs at low peptide concentrations, while higher CPP concentrations allow direct peptide penetration into the cell [40]. In contrast, direct penetration of penetratin has been shown to occur at low concentrations. Another factor influencing CPP internalization is the peptide net charge, especially, in the case of high positive charge determined by the presence of arginine residues. One more factor affecting peptide uptake is amphipathicity. Primary and secondary amphipathic peptides can directly cross the cell membrane at low concentrations, while non-amphipathic CPPs rely on endocytosis. The temperature at which the experiment is conducted also influences the mechanism of CPP internalization. Thus, direct translocation is observed at low temperatures (4°C), while endocytosis is more typical at higher temperatures (37°C). Transported molecules attached to the CPP can also greatly influence the uptake pathway. The size of the cargo molecule affects the size of the entire complex, resulting in the uptake mechanism different from that used for the internalization of CPP alone. The smaller the size, the greater the likelihood that the complex is internalized by direct translocation. Larger complexes are usually transported by endocytosis [40]. The efficiency of CPP uptake can also depend on the type of cells internalizing the peptide [58].
To summarize the above-mentioned, the mechanisms of CPP cellular uptake are diverse and depend on both the chemical nature of the peptide and properties of the cell membrane. The molecular mechanisms by which the structural changes in the local areas of the membrane can induce the peptide uptake remain to be elucidated. New data on the peptide interaction with cells are required for the development of efficient vector systems for the delivery of therapeutic molecules.
STRATEGIES FOR CPP APPLICATION
There are two main strategies for the CPP application for the delivery of therapeutic molecules (including nucleic acids) into cells.
The covalent strategy involves CPP conjugation with the cargo through a covalent bond. This strategy is suitable for the transport of neutrally charged molecules, for example, some proteins, peptides, peptide nucleic acids, etc. A promising direction in the covalent strategy is the use of the so-called degradable covalent bond between the CPP and cargo molecule. Such conjugate penetrates into the cell, after which it disintegrates with the release of the therapeutic molecule to the cytoplasm. An example is a disulfide bond-containing degradable linker between the TAT peptide and nucleic acids in [59]. In some cases, the covalent strategy is inapplicable, as the CPP conjugation with the therapeutic molecule can lead to the chemical modification of the latter, causing a decrease (or complete loss) of its biological activity. In addition, the covalent strategy is not suitable for the delivery of circular plasmid DNAs due to the lack of free valences for the conjugation with a CPP [60].
In the non-covalent strategy, the carrier peptide forms a complex with the cargo, e.g., due to electrostatic or hydrophobic interactions. This strategy is well suited for the delivery of therapeutic nucleic acids because CPPs often positively charged, and nucleic acids have a negative charge.
Application of CPPs for the nucleic acid delivery in preclinical studies. Numerous studies on the use of CPPs for the nucleic acid delivery into mammalian cells have been published. The vast majority of these works described in vitro experiments [43, 61, 62]. The application of CPPs for the delivery of nucleic acids is associated with a number of difficulties, mostly related to a relatively short half-life and lack of tissue and organ specificity of CPPs. Nevertheless, reports on the use of CPPs for the delivery of therapeutic nucleic acids in animal models of various diseases (inflammation, tumors, or infections) are published on a regular basis. In this section, we summarized the data on the use of CPPs in in vivo experiments (Table 2).
MPG-8, a truncated version of the amphipathic peptide MPG, was used to deliver siRNAs against mRNA for cyclin B1, a cell division regulatory protein whose expression is upregulated in various forms of cancer. MPG-8 was shown to form nanocomplexes with siRNA molecules. Intravenous administration of these complexes to mice with xenografted tumors caused a significant reduction in tumor size [63].
Michiue et al. [64] used TAT–DRBD obtained by fusing the TAT peptide with the double-stranded RNA-binding domain (DRBD) to deliver siRNA molecules. This hybrid peptide formed a complex with siRNAs via DRBD and penetrated into cells due to the TAT fragment, while transporting associated siRNA. This approach was tested in a mouse model of intracranial glioblastoma. The TAT–DRBD peptide delivered siRNA molecules against the tumorigenesis-related Egfr and Akt2 genes into cancer cells. Inactivation of these genes by siRNAs led to the apoptosis of tumor cells, which significantly promoted the survival of mice carrying the tumors [64].
A conjugate of the TAT carrier peptide and peptide nucleic acid (TAT–PNA-DR) directed against the genome of the hepatitis B virus (HBV) inhibited HBV replication in HepG2.2.15 cells. In the mouse model of HBV infection, administration of TAT–PNA-DR decreased the viral load by 80%. Moreover, TAT–PNA-DR showed low toxicity and immunogenicity, as well as the high stability in the serum [65].
A peptide carrier was also used to deliver phosphorodiamidate morpholino oligomers (PMOs, phosphorodiamidate morpholino oligomer) directed against genome of the herpes simplex virus type 1 (HSV-1). The PMO–peptide complex suppressed viral replication by 70-98% in vitro, while its local administration (10 μg) to the cornea of mice infected with HSV-1 reduced the incidence of disease by 37-50% [66].
Peptides are also used to deliver DNA vaccines. In particular, immunization of mice with the MPG peptide complex with the plasmid DNA coding for hepatitis C virus (HCV) antigens led to the appearance of specific IgG1 and IgG2a antibodies [67].
The conjugates of the (RXR)4XB peptide (where X is 6-aminohexanoic acid and B is β-alanine) with PMOs against dystrophin gene were successfully used in the models of Duchenne muscular dystrophy in mice and dogs (golden retriever muscular dystrophy) [68].
Since the cornea of the eye is one of the most commonly transplanted tissues, preclinical studies of the safety and efficacy of the AVI-5126 preparation were carried out in the rat corneal transplantation model. AVI-5126 is a conjugate of the (RXR)4 peptide with a PMO suppressing expression of the c-Myc transcription factor, a key factor in the transplant rejection. Storage of donor corneas before transplantation in AVI-5126 topical application of AVI-5126 after transplantation significantly reduced the graft rejection [69].
The T9(dR) peptide (where dR is D-arginine), a derivative of transportan, was used to deliver siRNAs directed against the influenza virus nucleoprotein gene. The complex reduced viral replication both in vitro and in the mouse model and prevented the death of infected mice [70].
The arginine-rich peptide P007 conjugated with PMO directed against the Coxsackie virus B3 genome demonstrated the antiviral effect both in vitro and in vivo [71]. Another arginine-rich peptide, R9F2, conjugated to PMO molecules directed against the Ebola virus genome, provided complete protection to mice administered with a lethal dose of the virus [72].
Our group has published a number of reports on the arginine- and lysine-rich cationic peptide dendrimer LTP. Due to the high content of arginine and lysine residues, LTP has a high positive charge and binds negatively charged nucleic acids [73]. We used this peptide to deliver siRNA molecules against genes encoding the proinflammatory cytokines IL-4 and IL-13 to the respiratory tract cells in mice with induced allergic rhinitis. Intranasal administration of the siRNA–LTP complex decreased production of the target cytokines by the cells of regional lymph nodes and reduced manifestations of the pathology, such as a decrease in the level of IgE antibodies, severity of the upper respiratory tract infiltration with proinflammatory cells, and remodeling of the respiratory tract [74]. In another study, LTP was used to deliver siRNA molecules against the Stat3 gene involved in the neutrophilic lung inflammation in bronchial asthma. It was shown that inhalation of this siRNA–LTP complex for 3 times led to a twofold suppression of the target gene expression in bronchoalveolar lavage (BAL) cells and a threefold decrease in the lung infiltration by neutrophils [75].
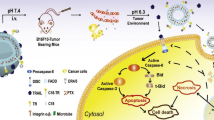
Another dendrimer (branched peptide), KK-46, was used as a carrier of siRNAs directed against the SARS-CoV-2 genomic RNA. Our team screened a library of siRNAs against different fragments of the SARS-CoV-2 genome for their ability to suppress virus reproduction. The most efficient siRNA was found to be against the genomic region encoding viral RNA-dependent RNA polymerase. It was modified by replacing five RNA nucleotides with LNA nucleotides, which significantly (3.7 times) increased the resistance of siRNA to the enzymatic degradation without affecting its antiviral activity. The siRNA–KK-46 complex suppressed viral replication up to 10,000 times in cultured cells. In the model of SARS-CoV-2 infection in Syrian hamsters, inhalation of the complex at a dose of 0.7-5.6 mg per kg body weight decreased the viral load in the lungs up to 200 times. Moreover, suppression of the virus replication in the lungs led to the improvement manifested as a reduction in the level of peribronchial infiltration by proinflammatory cells and decrease in the thickness of the bronchial walls. Preclinical safety studies established that the drug belongs to the class of low-toxic compounds [28].
Application of CPPs for the delivery of nucleic acids in clinical studies. CPPs can be used for the delivery of various types of molecules (proteins, peptides, cyclosporine, botulinum toxin, etc.) in medical practice. Several CPP-based preparations have reached the phase of clinical trials (see reviews by Guidotti et al. [62] and Stiltner et al. [77]); two of these preparations use CPPs for the delivery of therapeutic nucleic acids [27].
After successful completion of preclinical trials in the rat cornea transplantation model, AVI-5126 was tested in clinical studies for its safety and efficacy. This drug is the (RAhxR)4 peptide conjugated with the PMO targeting human transcription factor c-Myc. Suppression of this transcription factor helps to avoid rejection of the transplanted veins after coronary artery bypass surgery. In the trial, the excised vein was immersed in a solution containing 10 μM AVI-5126 before being implanted as a shunt [78]. However, the clinical trial was terminated and its results have not been published.
The world’s first etiotropic drug MIR 19 developed and approved for the treatment of COVID-19 infection is a complex of LNA-modified siRNA and KK-46 peptide carrier. In phase II clinical studies, the drug was administered by inhalation to the patients with moderately severe COVID-19 infection in hospital settings [17]. Patients in the control group (52 patients) received standard therapy according to the clinical recommendations of the Ministry of Health of the Russian Federation. The test group (52 patients) received MIR 19 by inhalation at a dose of 3.7 and 11.1 mg per day (these patients were deprived of any other etiotropic therapy). Clinical studies have shown that the drug was safe and well tolerated, and significantly reduces the time to clinical improvement (fever relief, normalization of respiratory rate, reduction of cough, and restoration of oxygen saturation to >95%) compared to the standard therapy [17]. Based on the clinical efficacy demonstrated in the study, the drug was registered for medical use (registration no. LP-007720).
To summarize, CPP-based nucleic acid delivery system have a great potential and significant prospects in clinical practice due to their safety and efficacy, ease of production, and low cost. Therapeutic nucleic acids delivered by CPP-based vectors are expected to enter the market in the nearest future.
CONCLUSION
Since the discovery of the TAT peptide in 1988, a large number of CPPs have been developed for the delivery of therapeutic agents, leading to the accumulation of significant experience of their in vitro and in vivo application.
The advantages of CPPs include a relative ease of production and storage and well-developed purification and standardization methods. Also, CPPs are biocompatible and nontoxic and typically cause no undesirable immune response because of their small size. The main limitations to the use of CPPs in medicine are low cell type specificity and short half-life.
However, these limitations can be overcome, first of all, by stabilization of the peptide structure via introduction of amino acid substitutions or creation of additional chemical bonds. Peptide dendrimerization (synthesis of branched peptides) can also increase the peptide stability due to the presence of unnatural ε-bonds. Greater stability can be also achieved by using unnatural amino acids or D-enantiomers, which are less sensitive to the enzymatic degradation than L-amino acids. The specificity of CPPs toward their target cells can be increased by binding these peptides to specific ligands with the formation of covalent or non-covalent complexes. Also, the targeted delivery can be achieved through the local administration of CPP-containing drugs, e.g., inhalation for the drug delivery to the lungs.
The use of CPPs for the delivery of various therapeutic molecules, including nucleic acids, has been tested in multiple preclinical. At least nine drugs have reached the stage of clinical trials, two of which used CPPs to deliver therapeutic nucleic acids. One of these drugs has been approved for medical use. All this indicates significant prospects of CPPs as the vehicle for the drug delivery in gene therapy.
Abbreviations
- ASO:
-
antisense oligonucleotide
- CPP:
-
cell-penetrating peptide
- DRBD:
-
double-stranded RNA-binding domain
- EGS:
-
external guide sequence
- LNA:
-
locked nucleic acid
- PMO:
-
phosphorodiamidate morpholino oligomer
- PNA:
-
peptide nucleic acid
- RISC:
-
RNA-induced silencing complex
- siRNA:
-
small interfering RNA
References
Chaudhary, N., Weissman, D., and Whitehead, K. A. (2021) mRNA vaccines for infectious diseases: principles, delivery and clinical translation, Nat. Rev. Drug Discov., 20, 817-838, https://doi.org/10.1038/S41573-021-00283-5.
Kulkarni, J. A., Witzigmann, D., Thomson, S. B., Chen, S., Leavitt, B. R., Cullis, P. R., and van der Meel, R. (2021) The current landscape of nucleic acid therapeutics, Nat. Nanotechnol., 16, 630-643, https://doi.org/10.1038/s41565-021-00898-0.
Talap, J., Zhao, J., Shen, M., Song, Z., Zhou, H., Kang, Y., Sun, L., Yu, L., Zeng, S., and Cai, S. (2021) Recent advances in therapeutic nucleic acids and their analytical methods, J. Pharm. Biomed. Anal., 206, 114368, https://doi.org/10.1016/J.JPBA.2021.114368.
Wang, Y., Zhang, R., Tang, L., and Yang, L. (2022) Nonviral delivery systems of mRNA vaccines for cancer gene therapy, Pharmaceutics, 14, 512, https://doi.org/10.3390/PHARMACEUTICS14030512.
Shirley, J. L., de Jong, Y. P., Terhorst, C., and Herzog, R. W. (2020) Immune responses to viral gene therapy vectors, Mol. Ther., 28, 709-722, https://doi.org/10.1016/J.YMTHE.2020.01.001.
Yan, Y., Liu, X. Y., Lu, A., Wang, X. Y., Jiang, L. X., and Wang, J. C. (2022) Non-viral vectors for RNA delivery, J. Control. Rel., 342, 241, https://doi.org/10.1016/J.JCONREL.2022.01.008.
Zou, Z., Shen, Q., Pang, Y., Li, X., Chen, Y., Wang, X., Luo, X., Wu, Z., Bao, Z., Zhang, J., Liang, J., Kong, L., Yan, L., Xiong, L., Zhu, T., Yuan, S., Wang, M., Cai, K., Yao, Y., Wu, J., Jiang, Y., Liu, H., Liu, J., Zhou, Y., Dong, Q., Wang, W., Zhu, K., Li, L., Lou, Y., Wang, H., Li, Y., and Lin, H. (2019) The synthesized transporter K16APoE enabled the therapeutic HAYED peptide to cross the blood-brain barrier and remove excess iron and radicals in the brain, thus easing Alzheimer’s disease, Drug Deliv. Transl. Res., 9, 394-403, https://doi.org/10.1007/s13346-018-0579-4.
Matijass, M., and Neundorf, I. (2021) Cell-penetrating peptides as part of therapeutics used in cancer research, Med. Drug Discov., 10, 100092, https://doi.org/10.1016/j.medidd.2021.100092.
Derakhshankhah, H., and Jafari, S. (2018) Cell penetrating peptides: a concise review with emphasis on biomedical applications, Biomed. Pharmacother., 108, 1090-1096, https://doi.org/10.1016/j.biopha.2018.09.097.
Kang, Y. C., Son, M., Kang, S., Im, S., Piao, Y., Lim, K. S., Song, M. Y., Park, K. S., Kim, Y. H., and Pak, Y. K. (2018) Cell-penetrating artificial mitochondria-targeting peptide-conjugated metallothionein 1A alleviates mitochondrial damage in Parkinson’s disease models, Exp. Mol. Med., 50, 1-13, https://doi.org/10.1038/s12276-018-0124-z.
Xie, J., Bi, Y., Zhang, H., Dong, S., Teng, L., Lee, R. J., and Yang, Z. (2020) Cell-penetrating peptides in diagnosis and treatment of human diseases: from preclinical research to clinical application, Front. Pharmacol., 11, 697, https://doi.org/10.3389/fphar.2020.00697.
Lopuszynski, J., Agrawal, V., and Zahid, M. (2022) Tissue-specific cell penetrating peptides for targeted delivery of small interfering RNAs, Med. Res. Arch., https://doi.org/10.18103/mra.v10i8.2998.
Nhàn, N. T. T., Maidana, D. E., and Yamada, K. H. (2023) Ocular delivery of therapeutic agents by cell-penetrating peptides, Cells, 12, 1071, https://doi.org/10.3390/cells12071071.
Zorko, M., Jones, S., and Langel, Ü. (2022) Cell-penetrating peptides in protein mimicry and cancer therapeutics, Adv. Drug Deliv. Rev., 180, 114044, https://doi.org/10.1016/J.ADDR.2021.114044.
Jiang, J. (2021) Cell-penetrating peptide-mediated nanovaccine delivery, Curr. Drug Targets, 22, 896-912, https://doi.org/10.2174/1389450122666210203193225.
Delcroix, M., and Riley, L. W. (2010) Cell-penetrating peptides for antiviral drug development, Pharmaceuticals, 3, 448-470, https://doi.org/10.3390/ph3030448.
Khaitov, M., Nikonova, A., Kofiadi, I., Shilovskiy, I., Smirnov, V., Elisytina, O., Maerle, A., Shatilov, A., Shatilova, A., Andreev, S., Sergeev, I., Trofimov, D., Latysheva, T., Ilyna, N., Martynov, A., Rabdano, S., Ruzanova, E., Savelev, N., Pletiukhina, I., Safi, A., Ratnikov, V., Gorelov, V., Kaschenko, V., Kucherenko, N., Umarova, I., Moskaleva, S., Fabrichnikov, S., Zuev, O., Pavlov, N., Kruchko, D., Berzin, I., Goryachev, D., Merkulov, V., Shipulin, G., Udin, S., Trukhin, V., Valenta, R., and Skvortsova, V. (2023) Treatment of COVID-19 patients with a SARS-CoV-2-specific siRNA-peptide dendrimer formulation, Allergy, 78, 1639-1653, https://doi.org/10.1111/ALL.15663.
Sridharan, K., and Gogtay, N. J. (2016) Therapeutic nucleic acids: current clinical status, Br. J. Clin. Pharmacol., 82, 659-672, https://doi.org/10.1111/BCP.12987.
Davies-Sala, C., Soler-Bistué, A., Bonomo, R. A., Zorreguieta, A., and Tolmasky, M. E. (2015) External guide sequence technology: a path to development of novel antimicrobial therapeutics, Ann. N. Y. Acad. Sci., 1354, 98-110, https://doi.org/10.1111/NYAS.12755.
Gadgil, H., and Jarrett, H. W. (2002) Oligonucleotide trapping method for purification of transcription factors, J. Chromatogr. A, 966, 99-110, https://doi.org/10.1016/s0021-9673(02)00738-0.
Vickers, T. A., Sabripour, M., and Crooke, S. T. (2011) U1 adaptors result in reduction of multiple pre-mRNA species principally by sequestering U1snRNP, Nucleic Acids Res., 39, e71, https://doi.org/10.1093/NAR/GKR150.
Agrawal, N., Dasaradhi, P. V. N., Mohmmed, A., Malhotra, P., Bhatnagar, R. K., and Mukherjee, S. K. (2003) RNA interference: biology, mechanism, and applications, Microbiol. Mol. Biol. Rev., 67, 657, https://doi.org/10.1128/MMBR.67.4.657-685.2003.
Hammond, S. M., Boettcher, S., Caudy, A. A., Kobayashi, R., and Hannon, G. J. (2001) Argonaute 2, a link between genetic and biochemical analyses of RNAi, Science, 293, 1146-1150, https://doi.org/10.1126/science.1064023.
Gangopadhyay, S., and Gore, K. R. (2022) Advances in siRNA therapeutics and synergistic effect on siRNA activity using emerging dual ribose modifications, RNA Biol., 19, 452-467, https://doi.org/10.1080/15476286.2022.2052641.
Wan-Hin Hui, R., Mak, L. Y., Seto, W. K., and Yuen, M. F. (2022) RNA interference as a novel treatment strategy for chronic hepatitis B infection, Clin. Mol. Hepatol., 28, 408, https://doi.org/10.3350/CMH.2022.0012.
Ray, K. K., Wright, R. S., Kallend, D., Koenig, W., Leiter, L. A., Raal, F. J., Bisch, J. A., Richardson, T., Jaros, M., Wijngaard, P. L. J., and Kastelein, J. J. P. (2020) Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol, New Eng. J. Med., 382, 1507-1519, https://doi.org/10.1056/nejmoa1912387.
Zhang, M. M., Bahal, R., Rasmussen, T. P., Manautou, J. E., and Zhong, X.-Bo (2021) The growth of siRNA-based therapeutics: updated clinical studies, Biochem. Pharmacol., 189, 114432, https://doi.org/10.1016/J.BCP.2021.114432.
Khaitov, M., Nikonova, A., Shilovskiy, I., Kozhikhova, K., Kofiadi, I., Vishnyakova, L., Nikolskii, A., Gattinger, P., Kovchina, V., Barvinskaia, E., Yumashev, K., Smirnov, V., Maerle, A., Kozlov, I., Shatilov, A., Timofeeva, A., Andreev, S., Koloskova, O., Kuznetsova, N., Vasina, D., Nikiforova, M., Rybalkin, S., Sergeev, I., Trofimov, D., Martynov, A., Berzin, I., Gushchin, V., Kovalchuk, A., Borisevich, S., Valenta, R., Khaitov, R., and Skvortsova, V. (2021) Silencing of SARS-CoV-2 with modified siRNA-peptide dendrimer formulation, Allergy, 76, 2840-2854, https://doi.org/10.1111/all.14850.
Pardi, N., Hogan, M. J., and Weissman, D. (2020) Recent advances in mRNA vaccine technology, Curr. Opin. Immunol., 65, 14-20, https://doi.org/10.1016/J.COI.2020.01.008.
Ledford, H. (2020) Moderna COVID vaccine becomes second to get US authorization, Nature, https://doi.org/10.1038/D41586-020-03593-7.
Blind, J. E., McLeod, E. N., Brown, A., Patel, H., and Ghosh, S. (2020) Biosafety practices for in vivo viral-mediated gene therapy in the health care setting, Appl. Biosaf., 25, 194, https://doi.org/10.1177/1535676020942195.
Roberts, T. C., Langer, R., and Wood, M. J. A. (2020) Advances in oligonucleotide drug delivery, Nat. Rev. Drug Discov., 19, 673, https://doi.org/10.1038/S41573-020-0075-7.
Chen, H., Ren, X., Xu, S., Zhang, D., and Han, T. (2022) Optimization of lipid nanoformulations for effective mRNA delivery, Int. J. Nanomed., 17, 2893-2905, https://doi.org/10.2147/IJN.S363990.
Paunovska, K., Loughrey, D., and Dahlman, J. E. (2022) Drug delivery systems for RNA therapeutics, Nat. Rev. Genet., 23, 265, https://doi.org/10.1038/S41576-021-00439-4.
Khan, M. M., Filipczak, N., and Torchilin, V. P. (2021) Cell penetrating peptides: A versatile vector for co-delivery of drug and genes in cancer, J. Controll. Rel., 330, 1220-1228, https://doi.org/10.1016/j.jconrel.2020.11.028.
Taylor, R. E., and Zahid, M. (2020) Cell penetrating peptides, novel vectors for gene therapy, Pharmaceutics, 12, 225, https://doi.org/10.3390/pharmaceutics12030225.
Falato, L., Gestin, M., and Langel, Ü. (2021) Cell-penetrating peptides delivering siRNAs: An overview, Methods Mol. Biol., 2282, 329-352, https://doi.org/10.1007/978-1-0716-1298-9_18.
Rádis-Baptista, G. (2021) Cell-penetrating peptides derived from animal venoms and toxins, Toxins (Basel), 13, 147, https://doi.org/10.3390/toxins13020147.
Zorko, M., and Langel, Ü. (2022) Cell-penetrating peptides, Methods Mol. Biol., 2383, 3-32, https://doi.org/10.1007/978-1-0716-1752-6_1.
Ruseska, I., and Zimmer, A. (2020) Internalization mechanisms of cell-penetrating peptides, Beilstein J. Nanotechnol., 11, 101-123, https://doi.org/10.3762/bjnano.11.10.
Holm, T., Andaloussi, S. El, and Langel, Ü. (2011) Comparison of CPP uptake methods, Methods Mol. Biol., 683, 207-217, https://doi.org/10.1007/978-1-60761-919-2_15.
Li, Z., Zhang, Y., Zhu, D., Li, S., Yu, X., Zhao, Y., Ouyang, X., Xie, Z., and Li, L. (2017) Transporting carriers for intracellular targeting delivery via non-endocytic uptake pathways, Drug Deliv., 24, 45-55, https://doi.org/10.1080/10717544.2017.1391889.
Sadeghian, I., Heidari, R., Sadeghian, S., Raee, M. J., and Negahdaripour, M. (2022) Potential of cell-penetrating peptides (CPPs) in delivery of antiviral therapeutics and vaccines, Eur. J. Pharm. Sci., 169, 106094, https://doi.org/10.1016/J.EJPS.2021.106094.
Trabulo, S., Cardoso, A. L., Mano, M., and de Lima, M. C. P. (2010) Cell-penetrating peptides – mechanisms of cellular uptake and generation of delivery systems, Pharmaceuticals, 3, 961-993, https://doi.org/10.3390/PH3040961.
Nath, A. (2021) Prediction for understanding the effectiveness of antiviral peptides, Comput. Biol. Chem., 95, 107588, https://doi.org/10.1016/j.compbiolchem.2021.107588.
Kaksonen, M., and Roux, A. (2018) Mechanisms of clathrin-mediated endocytosis, Nat. Rev. Mol. Cell Biol., 19, 313-326, https://doi.org/10.1038/NRM.2017.132.
Kawaguchi, Y., Takeuchi, T., Kuwata, K., Chiba, J., Hatanaka, Y., Nakase, I., and Futaki, S. (2016) Syndecan-4 is a receptor for clathrin-mediated endocytosis of arginine-rich cell-penetrating peptides, Bioconjug. Chem., 27, 1119-1130, https://doi.org/10.1021/ACS.BIOCONJCHEM.6B00082.
Futaki, S., and Nakase, I. (2017) Cell-surface interactions on arginine-rich cell-penetrating peptides allow for multiplex modes of internalization, Acc. Chem. Res., 50, 2449-2456, https://doi.org/10.1021/ACS.ACCOUNTS.7B00221.
Pujals, S., and Giralt, E. (2008) Proline-rich, amphipathic cell-penetrating peptides, Adv. Drug Deliv. Rev., 60, 473-484, https://doi.org/10.1016/J.ADDR.2007.09.012.
Säälik, P., Padari, K., Niinep, A., Lorents, A., Hansen, M., Jokitalo, E., Langel, Ü., and Pooga, M. (2009) Protein delivery with transportans is mediated by caveolae rather than flotillin-dependent pathways, Bioconjug. Chem., 20, 877-887, https://doi.org/10.1021/BC800416F.
Taylor, B. N., Mehta, R. R., Yamada, T., Lekmine, F., Christov, K., Chakrabarty, A. M., Green, A., Bratescu, L., Shilkaitis, A., Beattie, C. W., and das Gupta, T. K. (2009) Noncationic peptides obtained from azurin preferentially enter cancer cells, Cancer Res., 69, 537-546, https://doi.org/10.1158/0008-5472.CAN-08-2932.
Ye, J., Pei, X., Cui, H., Yu, Z., Lee, H., Wang, J., Wang, X., Sun, L., He, H., and Yang, V. C. (2018) Cellular uptake mechanism and comparative in vitro cytotoxicity studies of monomeric LMWP-siRNA conjugate, J. Industr. Engineer. Chem., 63, 103-111, https://doi.org/10.1016/J.JIEC.2018.02.005.
Yang, S. T., Zaitseva, E., Chernomordik, L. V., and Melikov, K. (2010) Cell-penetrating peptide induces leaky fusion of liposomes containing late endosome-specific anionic lipid, Biophys. J., 99, 2525-2533, https://doi.org/10.1016/J.BPJ.2010.08.029.
Cerrato, C. P., and Langel, Ü. (2022) An update on cell-penetrating peptides with intracellular organelle targeting, Expert Opin. Drug Deliv., 19, 133-146, https://doi.org/10.1080/17425247.2022.2034784.
Wang, H. Y., Chen, J. X., Sun, Y. X., Deng, J. Z., Li, C., Zhang, X. Z., and Zhuo, R. X. (2011) Construction of cell penetrating peptide vectors with N-terminal stearylated nuclear localization signal for targeted delivery of DNA into the cell nuclei, J. Control Rel., 155, 26-33, https://doi.org/10.1016/J.JCONREL.2010.12.009.
Alkhashrom, S., Kicuntod, J., Stillger, K., Lützenburg, T., Anzenhofer, C., Neundorf, I., Marschall, M., and Eichler, J. (2022) A peptide inhibitor of the human cytomegalovirus core nuclear egress complex, Pharmaceuticals, 15, 1040, https://doi.org/10.3390/PH15091040.
Huang, S., Zhu, Z., Jia, B., Zhang, W., and Song, J. (2021) Design of acid-activated cell-penetrating peptides with nuclear localization capacity for anticancer drug delivery, J. Pept. Sci., 27, e3354, https://doi.org/10.1002/PSC.3354.
Mueller, J., Kretzschmar, I., Volkmer, R., and Boisguerin, P. (2008) Comparison of cellular uptake using 22 CPPs in 4 different cell lines, Bioconjug. Chem., 19, 2363-2374, https://doi.org/10.1021/BC800194E.
Chiu, Y. L., Ali, A., Chu, C. Y., Cao, H., and Rana, T. M. (2004) Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells, Chem. Biol., 11, 1165-1175, https://doi.org/10.1016/j.chembiol.2004.06.006.
Tai, W., and Gao, X. (2017) Functional peptides for siRNA delivery, Adv. Drug Deliv. Rev., 110-111, 157-168, https://doi.org/10.1016/J.ADDR.2016.08.004.
Wang, F., Wang, Y., Zhang, X., Zhang, W., Guo, S., and Jin, F. (2014) Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery, J. Control Rel., 174, 126-136, https://doi.org/10.1016/J.JCONREL.2013.11.020.
Guidotti, G., Brambilla, L., and Rossi, D. (2017) Cell-penetrating peptides: from basic research to clinics, Trends Pharmacol. Sci., 38, 406-424, https://doi.org/10.1016/J.TIPS.2017.01.003.
Crombez, L., Morris, M. C., Dufort, S., Aldrian-Herrada, G., Nguyen, Q., Mc Master, G., Coll, J. L., Heitz, F., and Divita, G. (2009) Targeting cyclin B1 through peptide-based delivery of siRNA prevents tumour growth, Nucleic Acids Res., 37, 4559-4569, https://doi.org/10.1093/NAR/GKP451.
Michiue, H., Eguchi, A., Scadeng, M., and Dowdy, S. F. (2009) Induction of in vivo synthetic lethal RNAi responses to treat glioblastoma, Cancer Biol. Ther., 8, 2304-2311, https://doi.org/10.4161/CBT.8.23.10271.
Zeng, Z., Han, S., Hong, W., Lang, Y., Li, F., Liu, Y., Li, Z., Wu, Y., Li, W., Zhang, X., and Cao, Z. (2016) A Tat-conjugated peptide nucleic acid Tat-PNA-DR inhibits hepatitis B virus replication in vitro and in vivo by targeting LTR direct repeats of HBV RNA, Mol. Ther. Nucleic Acids, 5, e295, https://doi.org/10.1038/MTNA.2016.11.
Moerdyk-Schauwecker, M., Stein, D. A., Eide, K., Blouch, R. E., Bildfell, R., Iversen, P., and Jin, L. (2009) Inhibition of HSV-1 ocular infection with morpholino oligomers targeting ICP0 and ICP27, Antiviral Res., 84, 131-141, https://doi.org/10.1016/J.ANTIVIRAL.2009.07.020.
Mehrlatifan, S., Mirnurollahi, S. M., Motevalli, F., Rahimi, P., Soleymani, S., and Bolhassani, A. (2016) The structural HCV genes delivered by MPG cell penetrating peptide are directed to enhance immune responses in mice model, Drug Deliv., 23, 2852-2859, https://doi.org/10.3109/10717544.2015.1108375.
Moulton, H. M., Fletcher, S., Neuman, B. W., McClorey, G., Stein, D. A., Abes, S., Wilton, S. D., Buchmeier, M. J., Lebleu, B., and Iversen, P. L. (2007) Cell-penetrating peptide-morpholino conjugates alter pre-mRNA splicing of DMD (Duchenne muscular dystrophy) and inhibit murine coronavirus replication in vivo, Biochem. Soc. Trans., 35, 826-828, https://doi.org/10.1042/BST0350826.
Hosseini, A., Lattanzio, F. A., Samudre, S. S., Disandro, G., Sheppard, J. D., and Williams, P. B. (2012) Efficacy of a phosphorodiamidate morpholino oligomer antisense compound in the inhibition of corneal transplant rejection in a rat cornea transplant model, J. Ocul. Pharmacol. Ther., 28, 194-201, https://doi.org/10.1089/JOP.2011.0135.
Zhang, C., Ren, W., Liu, Q., Tan, Z., Li, J., and Tong, C. (2019) Transportan-derived cell-penetrating peptide delivers siRNA to inhibit replication of influenza virus in vivo, Drug Des. Devel. Ther., 13, 1059-1068, https://doi.org/10.2147/DDDT.S195481.
Yuan, J., Stein, D. A., Lim, T., Qiu, D., Coughlin, S., Liu, Z., Wang, Y., Blouch, R., Moulton, H. M., Iversen, P. L., and Yang, D. (2006) Inhibition of Coxsackievirus B3 in cell cultures and in mice by peptide-conjugated morpholino oligomers targeting the internal ribosome entry site, J. Virol., 80, 11510, https://doi.org/10.1128/JVI.00900-06.
Enterlein, S., Warfield, K. L., Swenson, D. L., Stein, D. A., Smith, J. L., Gamble, C. S., Kroeker, A. D., Iversen, P. L., Bavari, S., and Mühlberger, E. (2006) VP35 knockdown inhibits Ebola virus amplification and protects against lethal infection in mice, Antimicrob. Agents Chemother., 50, 984, https://doi.org/10.1128/AAC.50.3.984-993.2006.
Kozhikhova, K. V., Shilovskiy, I. P., Shatilov, A. A., Timofeeva, A. V., Turetskiy, E. A., Vishniakova, L. I., Nikolskii, A. A., Barvinskaya, E. D., Karthikeyan, S., Smirnov, V. V., Kudlay, D. A., Andreev, S. M., and Khaitov, M. R. (2020) Linear and dendrimeric antiviral peptides: design, chemical synthesis and activity against human respiratory syncytial virus, J. Mater. Chem. B, 8, 2607-2617, https://doi.org/10.1039/c9tb02485a.
Shilovskiy, I., Nikonova, A., Barvinskaia, E., Kaganova, M., Nikolskii, A., Vishnyakova, L., Kovchina, V., Yumashev, K., Korneev, A., Petukhova, O., Kudlay, D., Smirnov, V., Andreev, S., Kozhikhova, K., Shatilov, A., Shatilova, A., Maerle, A., Sergeev, I., Trofimov, D., and Khaitov, M. (2022) Anti-inflammatory effect of siRNAs targeted il-4 and il-13 in a mouse model of allergic rhinitis, Allergy, 77, 2829-2832, https://doi.org/10.1111/ALL.15366.
Nikolskii, A. A., Shilovskiy, I. P., Yumashev, K. V., Vishniakova, L. I., Barvinskaia, E. D., Kovchina, V. I., Korneev, A. V., Turenko, V. N., Kaganova, M. M., Brylina, V. E., Nikonova, A. A., Kozlov, I. B., Kofiadi, I. A., Sergeev, I. V., Maerle, A. V., Petukhova, O. A., Kudlay, D. A., and Khaitov, M. R. (2021) Effect of local suppression of Stat3 gene expression in a mouse model of pulmonary neutrophilic inflammation, Immunologiya, 42, 600-614, https://doi.org/10.33029/0206-4952-2021-42-6-600-614.
Shilovskiy, I., Sundukova, M., Korneev, A., Nikolskii, A., Barvinskaya, E., Kovchina, V., Vishniakova, L., Turenko, V., Yumashev, K., Kaganova, M., Brilina, V., Sergeev, I., Maerle, A., Kudlay, D., Petukhova, O., Khaitov, M. (2022) The mixture of siRNAs targeted to IL-4 and IL-13 genes effectively reduces the airway hyperreactivity and allergic inflammation in a mouse model of asthma, Int. Immunopharmacol., 103, 108432, https://doi.org/10.1016/J.INTIMP.2021.108432.
Stiltner, J., McCandless, K., and Zahid, M. (2021) Cell-penetrating peptides: applications in tumor diagnosis and therapeutics, Pharmaceutics, 13, 890, https://doi.org/10.3390/PHARMACEUTICS13060890.
Abes, R., Arzumanov, A. A., Moulton, H. M., Abes, S., Ivanova, G. D., Iversen, P. L., Gait, M. J., and Lebleu, B. (2007) Cell-penetrating-peptide-based delivery of oligonucleotides: an overview, Biochem. Soc. Trans., 35, 775-779, https://doi.org/10.1042/BST0350775.
Funding
The study was supported by the Russian Science Foundation (project no. 22-25-00182; https://rscf.ru/project/22-25-00182/ [in Russian]).
Author information
Authors and Affiliations
Contributions
M.R.Kh. developed the concept and supervised the work; E.D.T. wrote the text of the article; I.P.Sh. wrote and edited the article.
Corresponding author
Ethics declarations
The authors declare no conflict of interest. The authors declare no conflicts of interest. This article does not contain description of studies with the involvement of humans or animal subjects.
Rights and permissions
Open access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Timotievich, E.D., Shilovskiy, I.P. & Khaitov, M.R. Cell-Penetrating Peptides as Vehicles for Delivery of Therapeutic Nucleic Acids. Mechanisms and Application in Medicine. Biochemistry Moscow 88, 1800–1817 (2023). https://doi.org/10.1134/S0006297923110111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923110111