Abstract
Chronic kidney disease can progress to the end-stage renal disease (ESRD) characterized by a high risk of morbidity and mortality. ESRD requires immediate therapy or even dialysis or kidney transplantation, therefore, its timely diagnostics is critical for many patients. ESRD is associated with pathological changes, such as inflammation, fibrosis, endocrine disorders, and epigenetic changes in various cells, which could serve as ESRD markers. The review summarizes information on conventional and new ESRD biomarkers that can be assessed in kidney tissue, blood, and urine. Some biomarkers are specific to a particular pathology, while others are more universal. Here, we suggest several universal inflammatory, fibrotic, hormonal, and epigenetic markers indicative of severe deterioration of renal function and ESRD progression for improvement of ESRD diagnostics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Chronic kidney disease (CKD) is characterized by a gradual loss of kidney function and involves a series of local and systemic pathological processes, from decrease in glomerular filtration rate (GFR) to the development of end-stage renal disease (ESRD) [1-3]. CKD and particularly ESRD are important factors in the deterioration of patients’ quality of life and mortality. The mortality rate in ESRD patients is significantly higher than in patients with CKD only (in the absence of ESRD) and varies from 20 to 50% over 24 months even when modern hemodialysis techniques are used [4]. The late CKD stages require renal replacement therapy, such as peritoneal dialysis, hemodialysis, or kidney transplantation. However, allograft transplantation is available only for 10% patients needing new organs [5]. By the end of 2018, the number of people with ESRD exceeded 785,000 in the United States [6] and the number of patients diagnosed with this pathology has been steadily increasing [4].
At present, assessment of CKD stage and diagnostics of ESRD are based on morphological and functional symptoms of kidney damage. The definition and classification of CKD was proposed by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) in 2002 and approved by the Kidney Disease: Improving Global Outcomes organization (KDIGO) in 2004 [7]. According to KDIGO 2012 clinical practice guideline [8], CKD classification is based on the estimated GFR (eGFR) (Fig. 1): stage 1 – normal eGFR (above 90 ml/min/1.73 m2) with microalbuminuria; stage 2 – eGFR, 60-89 ml/min/1.73 m2 with microalbuminuria; stage 3 – eGFR, 30-59 ml/min/1.73 m2; stage 4 – eGFR, 15-29 ml/min/1.73 m2; stage 5/ESRD – eGFR, below 15 ml/min/1.73 m2. Stages 3-5 are defined as CKD.
Clinical practice uses several approaches to the GFR assessment, including those based on measuring the clearance of endogenous creatinine and cystatin C (eGFR) or clearance of exogenous substances, such as radiopharmaceuticals, e.g., 99mTc-labeled diethylenetriamine pentaacetate (measured GFR, mGFR) [9, 10]. mGFR is a more accurate parameter for GFR assessment, but its evaluation requires special equipment and permission to work with radiopharmaceuticals, which significantly limits the use of this method.
Recently, using eGFR as a single parameter in the diagnostics of CKD has been criticized and found insufficient, as it has the following limitations: (i) kidney is a multifunctional organ, while GFR reflects only one of its functions; (ii) various non-renal factors can influence GFR measurements; (iii) GFR has intrinsic variability and depends on food and fluid intake, cardiovascular status, and blood pressure, especially in the case of impaired autoregulation or use of medications; (iv) GFR changes with age in a unique nonlinear pattern; (v) eGFR may not correlate with GFR under certain conditions and in certain diseases. Therefore, eGFR can be used as an initial screening tool, but should not be applied to the diagnostics of CKD without careful assessment of the entire clinical profile [11]. Therefore, there is still a need for biomarkers that can be employed for the diagnostics of CKD and, especially, ESRD.
Renal fibrosis is a typical outcome of inflammation that occurs in almost all nephropathies [12, 13]. Fibrosis can affect all kidney compartments, ultimately causing destruction of the renal parenchyma and ESRD development. Therefore, markers of kidney fibrosis can also be used as universal markers in the diagnostics of CKD and ESRD. Renal failure is also accompanied by endocrine disorders and epigenetic changes in various cells, which can serve as ESRD biomarkers as well. In this review, we summarize information on currently known biomarkers of inflammation, kidney fibrosis, hormonal changes, and epigenetic changes and discusses their application in the diagnostics of CKD-to-ESRD progression.
ESRD PROGRESSION
The most common cause of CKD and ESRD is diabetes mellitus [14]. Other causes [4, 15] are hypertension, renal vascular disease, primary or secondary glomerulonephritis, cystic kidney disease, tubulointerstitial nephritis, obstruction or dysfunction of the urinary tract, recurrent kidney stone disease, congenital kidney or bladder defects, acute kidney injury, autoimmune diseases, nephrotoxins, obesity, and certain medications, including nonsteroidal anti-inflammatory drugs, calcineurin inhibitors, and antiretroviral drugs.
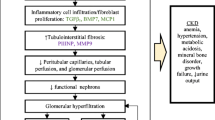
All these diseases are characterized by changes in renal function, reflecting the contribution of individual nephrons to the total GFR [4]. A decline in the renal function can be initially asymptomatic and cause hyperfiltration in nephrons. Together with the compensatory nephron hypertrophy, hyperfiltration allows kidneys to maintain the GFR. As a result, a patient with mild renal dysfunction can have normal creatinine levels, so that the disease can remain undetected for some time [16]. Sooner or later, this adaptive mechanism exhausts itself, which eventually results in the damage of glomeruli of remaining nephrons [4]. An increased glomerular capillary pressure damages the capillaries, leading to focal and segmental glomerulosclerosis and then to global glomerulosclerosis. Pathomorphological alterations characteristic of ESRD are shown in Fig. 2.
One of the most important factors in the development of renal pathology is inflammation [15, 17, 18]. ESRD can also accompany glomerulosclerosis, tubulointerstitial fibrosis, and atrophy [15, 19]. Finally, ESRD is associated with endocrine disorders and epigenetic changes.
INFLAMMATORY MARKERS
Chronic inflammation plays an important role in the development of renal diseases and can be used as a prognostic factor in the all-cause and cardiovascular mortality in hemodialysis patients [20]. Inflammatory response involves multiple molecules, including pro-inflammatory cytokines, chemokines, cell adhesion molecules, and various growth and nuclear factors [21].
There have been a number of studies on the efficacy of inflammatory markers in the detection of deteriorating kidney function. It was found that when used in combination with the evaluation of creatinine content, inflammatory and prothrombotic markers can serve as predictors of changes in the renal function in elderly patients without direct measurements of GFR [22]. At the same time, no associations between nine inflammatory and procoagulant markers [C-reactive protein, interleukin-6 (IL-6), intercellular adhesion molecule 1, white blood cell count, fibrinogen, factor VII, factor VIII, D-dimer, plasmin–antiplasmin complex) and rapid decline in the renal function has been revealed [23]. The only parameter consistently associated with eGFR (estimated with cystatin C) was a decrease in the baseline level of serum albumin [23].
It was shown that CKD (in particular, CKD stage 5) is accompanied by the elevation of plasma IL-6, which could be used for the prediction of the all-cause and cardiovascular mortality in pre-dialysis patients with CKD stages 2-5 [20]. Moreover, IL-6 plasma levels as predictors of mortality ensured more precise results than the other three major inflammatory biomarkers, namely, C-reactive protein, TNF-α, and serum albumin [20]. The data on the association of C-reactive protein (common inflammatory biomarker) with eGFR and its use for the prediction of eGFR decline are contradictory [24-26]. It was also found that the concentration of IL-18 negatively correlates with the creatinine clearance, which was masked by continuous ambulatory peritoneal dialysis [27].
Analysis of potential inflammatory biomarkers and kidney injury biomarkers in diabetic kidney disease highlighted the controversial results in cohort studies of the predictive ability of tumor necrosis factor receptors (TNFRs) for ESRD in diabetes [28]. In IgA nephropathy, circulating TNFRs reflected histological changes and clinical severity of the disease and negatively correlated with eGFR. Moreover, elevated serum concentrations of TNFRs during the disease onset served as early biomarkers of subsequent renal progression in IgA nephropathy patients [29]. Seventeen circulating inflammatory proteins, many of the belonging to the TNFR superfamily, were proposed as predictors of a 10-year risk of ESRD in type 1 and type 2 diabetes [30].
The following biomarkers of systemic inflammatory response identified in blood were found to be closely associated with CKD progression [18]:
-
proinflammatory cytokines: TNF-α, IL-6, and IL-18;
-
chemokines: IL-8 (CXCL8), IL-34, stromal cell-derived factor 1α (SDF1α/CXCL12), monocyte chemoattractant protein-1 (MCP-1/CCL2), and macrophage inflammatory protein 1β (MIP-1β/CCL4);
-
growth factors: GM-CSF (granulocyte-macrophage colony-stimulating factor), fibroblast growth factor (FGF) 23, and HGF (hepatocyte growth factor);
-
soluble forms of receptors: sTNFR1 and sTNFR2, soluble CD40 ligand (sCD40L), and sCD163 (SR-I3);
-
cyclophilin A.
Unfortunately, there is a limited information on the relationship between inflammatory markers, GFR levels, and ESRD development at different CKD stages, including patients on dialysis. Recently, it was shown that the circulating adhesion molecule VCAM-1 is inversely associated with eGFR and can predict eGFR decline in a general population [26]. Another inflammatory marker measured in CKD was activity of serum adenosine deaminase [31] which negatively correlated with eGFR [31], although the authors noted that using only one parameter of renal function would be insufficient for ESRD evaluation.
Soluble molecules are not the only inflammatory biomarkers. Thus, in situ inflammatory status and lymphocyte immune phenotype [32, 33] can be used for distinguishing ESRD patients from the control group. Reduction in the number of regulatory T cells (Tregs) can contribute to the ESRD development in systemic lupus erythematosus. Also, ESRD patients with a low Treg count have shown a shift in the cytokine profile from the anti-inflammatory toward proinflammatory cytokines, as well as higher concentration of antibodies against human leukocyte antigen in peripheral blood [32]. Confocal microscopy of biopsies from patients with lupus nephritis showed that the high count of B cells correlated with the resistance to ESRD development, while high amounts of CD8+, γδ, and other CD4–CD8– T cells were associated with acute renal failure and ESRD progression [33]. The differences in the phenotypic patterns of the T- and B-cell populations were observed between ESRD patients and healthy controls. ESRD patients demonstrated reduction in the number of naïve CD4 subpopulations, CD19+IgD+CD27+ cells, and CD8+PD1+ cells, along with the increase in the content of senescent cell subpopulations [34].
Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) can also have a diagnostic significance in the assessment of inflammation in ESRD patients. NLR was found to be associated with inflammation in ESRD patients, including both hemodialysis and peritoneal dialysis patients [35-37], and with the estimated survival of hemodialysis patients [38, 39]. It was demonstrated that PLR is also associated with inflammation and can be used to predict mortality in hemodialysis patients [37, 38]. A 7-year cohort study of non-dialysis ESRD patients revealed that both NLR and PLR were related to inflammation, as demonstrated by their association with C-reactive protein [40].
Since ESRD development is a unique process observed in different pathologies, the use of inflammatory markers in renal failure seems contradictory and has to be combined with other diagnostic approaches.
FIBROSIS MARKERS
Healthy tissues are characterized by a balance between the synthesis and proteolysis of extracellular matrix (ECM) proteins (collagen, fibronectin, laminin, etc.). Disturbances of this balance can induce uncontrolled deposition of ECM components, ultimately impairing organ morphology and/or function and leading to fibrosis development [12]. This process can occur in all organs and causes almost 45% deaths in developed countries [41]. Since 1990s, the prevalence of CKD, which is accompanied by renal fibrosis, has increased and reached 1.2 million deaths worldwide in 2017 [42].
Excessive deposition of ECM in renal fibrosis results in the damage and replacement of functional parenchyma, leading to kidney failure [13]. This pathological process can affect three different renal compartments: glomeruli (causing glomerulosclerosis), tubulointerstitium (interstitial fibrosis), and vasculature (arteriosclerosis and perivascular fibrosis) [43]. Regardless of the cause and origin of fibrosis, its histological pattern in different kidney diseases is essentially the same. Hence, kidney fibrosis markers can be considered as universal molecules for the diagnostics of CKD and ESRD.
Collagens. Collagens are the most common markers of fibrosis. Renal interstitium contains collagens I, II, III, IV, VI, VII, and XV [44]. Type I collagen is the main component of fibrous tissues in all organs [44, 45]. Collagens II and III are believed to accumulate at the early stages of kidney fibrosis development [46]. An increase in the content of collagens IV and VI in kidney fibrous tissue has also been demonstrated [47, 48]. Type IV collagen is a component of the basement membrane and is used as a marker of glomerulosclerosis and interstitial fibrosis [49, 50].
The most common method for fibrosis identification in clinical and experimental studies is staining the biopsies with Masson’s trichrome or Picrosirius Red. However, despite the widespread use of Masson’s staining, it has poor specificity to collagen itself and reflects interstitial volume rather than fibrosis per se [51]. Picrosirius Red is more suitable for fibrillar collagen staining, but it also has its limitations, in particular, the lack of specificity to collagen IV, which prevents the use of this stain in the analysis of tubular basement membrane thickening or diagnostics of glomerulosclerosis [51]. Immunohistochemical assays lack the above-mentioned limitations and can directly reveal the content and tissue location of collagens, allowing evaluation of the degree of kidney fibrosis by morphometric measurements [52, 53].
Beside histological and immunohistochemical methods, the content of collagen can be evaluated by the biochemical analysis of hydroxyproline [51], because collagen is one of the few proteins containing this amino acid. The percentage of hydroxyproline in the amino acid composition of collagen is constant in most mammalian organs [54, 55].
The most available biological samples in clinical and experimental studies are serum and urine, which can be assayed for the presence of collagen fragments (Table 1). Hence, collagen or its fragments can be assessed in the urine or blood samples as biomarkers of renal failure and, in particular, ESRD, using noninvasive methods. This approach can be employed to investigate the dynamics of renal failure development. For instance, the study on the relation between the GFR and levels of collagen III fragments reflecting its active formation (PRO-C3) and degradation (C3M) has shown that the C3M/creatinine ratio in urine increased with CKD development [56]. The PRO-C3/creatinine ratio in urine and serum increases only at stages 4 and 5 [56]. And vice versa, low C3M/creatinine ratio in the urine and increased serum content of PRO-C3 were associated with an increased risk of developing ESRD and death [56]. However, no association between the urine and serum levels of C3M and mortality in the diabetic kidney disease was found [57].
Comparison of healthy people and patients with CKD based on the data of the Human Urinary Proteome Database has shown that collagen Ia1 fragments can be used for the diagnostics of kidney failure. Sixty-three out of 707 urinary collagen Ia1 fragments positively associated with the GFR; 6 fragments negatively associated with the GFR [58].
Analysis of collagens and collagen fragments by immunoassay methods is even more useful for the clinical diagnostics of renal failure. Thus, the content of collagen IV in the urine of young patients with type 1 diabetes correlated with a decreased GFR, although no association with ESRD has been found [59]. The same results were obtained for patients with type 2 diabetes [60]. An increase in the serum level of PRO-C6 (fragment reflecting collagen VI active formation) showed association with the GFR decline, ESRD development, and mortality in patients with type 1 diabetes, while higher urinary levels of PRO-C6, on the contrary, correlated with a reduced risk of GFR reduction [61]. Serum and urine levels of endotrophin (collagen VI fragment) can be used for prediction of ESRD development in CKD patients [62, 63] and were chosen as indicators (instead of eGFR) in the multivariable model of CKD-related mortality due to their higher predictive power [62]. Urinary endotrophin/creatinine ratio was found to be associated with one-year CKD progression, independently of traditional risk factors, such as eGFR, age, sex, and urinary albumin/creatinine excretion ratio [63].
It has been shown that using a combination of biomarkers (sLG1M, sPRO-C3, sPRO-C6, uPRO-C3/creatinine ratio, uPRO-C6/creatinine ratio, uC3M/creatinine ratio) can improve prediction of renal outcome (sensitivity 50.0%; specificity 77.8%; Area under the curve (AUC) 0.806) compared to the clinical parameters, such as eGFR and proteinuria (sensitivity 45.2%; specificity 87.3%; AUC 0.751) [64]. Therefore, a combination of GFR and ECM components as fibrosis biomarkers may improve prediction of CKD progression and renal outcome.
ECM and intermediate filament as biomarkers. Beside collagen, ECM contains fibronectin and thrombospondin 1 (TSP-1). Intermediate filament proteins (vimentin, nestin) are associated with kidney fibrosis (Table 2). Some of them can be used for the diagnostics of renal failure, as their levels correlate with the GFR. Thus, the content of fibronectin in the urine of patients with the Bardet–Biedl syndrome correlates with eGFR [74]. Detection of vimentin mRNA in urine can also be useful in the diagnostics of renal failure because of its negative correlation with GFR in patients with CKD [75].
Other kidney fibrosis markers. Non-ECM proteins can also be used in the diagnostics of kidney fibrosis (Table 3), as some of them are associated with the GFR and can be helpful in ESRD prognosis in renal diseases and after kidney transplantation.
High TGF-β1/BMP7 ratio can indicate ESRD development in patients with type 2 diabetes [89]. Another potential biomarker of kidney failure is galectin 3 due to its correlation with the GFR in patients with diabetic kidney disease [117]. In the same patients, identification of C-X-C motif chemokine ligand 16 (CXCL16) in urine indicates fibrosis development [72], while the plasma levels of this cytokine reflect GFR and CKD transition to ESRD [118, 119]. The content of CXCL16 in CKD patients with type 2 diabetes mellitus is higher than in CKD patients with no diabetes [118]; therefore, the serum and/or urine levels of CXCL16 can serve as prognostic/diagnostic markers for the severity of CKD and its progression to ESRD. Similarly, the content of endostatin in the plasma is negatively associated with the GFR [120, 121] and reflects CKD development; it can also predict mortality in patients with type 2 diabetes [122, 123] or kidney transplant damage [122].
The plasma content of monocyte chemoattractant protein 1 (MCP-1) can reflect the development of fibrosis, GFR decline, and mortality, in CKD [124]. Other fibrosis markers associated with the development of renal failure and GFR in CKD include retinol-binding proteins (RBP) in urine [125], connective tissue growth factor (CTGF) in plasma [126], and human epididymal secretory protein 4 (HE4) in serum [127]. The content of RBP and the DcR2/creatinine ratio in urine can be used for the diagnostics of IgA nephropathy [93, 128]. The plasma level of CTGF and upregulated expression of its mRNA in kidneys reflect the stages of CKD in patients with lupus nephritis [129]. Serum FGF-2 negatively correlates with the GFR and proteinuria in patients with glomerulonephritis.
The risk of ESRD development in elderly patients can be predicted by measuring the serum level of uromodulin [130], whereas blood levels of uromodulin reflect the development of renal failure after kidney transplantation [116]. The EGF/creatinine ratio in the urine of patients after transplantation correlates with a GFR decline [116]. Identification of interstitial fibrosis with Masson’s trichrome staining together with the levels of α-smooth muscle actin (α-SMA) in biopsies, can accurately predict the development of renal allograft chronic dysfunction [131].
Some of the above-described fibrosis biomarkers have more predictive power in the case of CKD progression and loss of renal function than the GFR. For example, urinary MCP-1/creatinine levels have been used to diagnose inflammatory activity in kidneys in male patients, even when the eGFR values were normal. MCP-1 measurements can reveal renal inflammation early in the development of the Alport syndrome [132]. In IgA nephropathy, urinary MMP-7 levels can serve as an independent and powerful predictor of disease progression, even in the early-stage patients with eGFR of ≥60 ml/min/1.73 m2 [133]. Thus, the use of urinary MMP-7 content together with other parameters, such as eGFR, mean arterial blood pressure, proteinuria, and histological score, has significantly improved prediction of a 3-year risk of IgA nephropathy progression [133]. Hence, kidney fibrosis biomarkers can reflect the severity of CKD and its progression to ESRD, as well as to predict kidney transplant rejection and even mortality.
EPIGENETIC MARKERS
Involvement of epigenetic processes in the development of renal fibrosis has been repeatedly demonstrated in cell cultures, animal models, and patients with diabetic nephropathy, ischemia, lupus nephritis, and other pathologies [134-137]. Epigenetic changes can directly mediate transition from acute injury to chronic kidney disease [138-141]. It was suggested that epigenetic markers could predict the severity of CKD, distinguish between different CKD stages, and evaluate the risk of ESRD. The most promising epigenetic markers of renal fibrosis are microRNAs (miRs) and DNA methylation; they are commonly analyzed in blood cells or urine and used as prognostic factors of CKD and ESRD development.
miRs. miRs are small non-coding RNAs encoded in gene introns or transcribed independently. The primary transcripts are processed into mature miRs that suppress the activity of various genes through RNA interference. At the same time, the effect of individual miRs can be very broad, as almost every miR has more than 100 targets, and depends on the type and state of affected cells. miRs have been widely studied in various models of renal pathologies, and many miRs involved in the development of renal fibrosis have been identified [142]. For instance, miR-21, miR-92, and miR-122 can be used for evaluating the stages of CKD and risks of ESRD development. The main effects of miR-21 involve the shift of cellular metabolism to glycolysis. miR-21 increases cell viability during acute stress by downregulating many genes, such as its direct targets peroxisome proliferator-activated receptor α (PPARα), acyl-coenzyme A oxidase 1 (ACOX1), coenzyme A synthase, pyruvate dehydrogenase, and pyruvate carboxylase. miR-21 also represses the genes of Mpv17-like proteins that regulate redox metabolic pathways [143-150]. There are other miRs associated with renal pathologies, but their diagnostic value in the case of ESRD still has to be established. The data of miRs potentially involved in CKD and ESRD are presented in Table 4.
DNA methylation. Due to the revealed correlation between the DNA methylation level and kidney pathologies, DNA methylation can serve as a biomarker for CKD or ESRD severity and risk of their development. One of the markers of DNA methylation is 5-methyl-2′-deoxycytidine (5-Me-dC), whose content is increased in the urine of patients with late-stage CKD. When accompanied by macroalbuminuria or appearance of α1-microglobulin (α1m) in urine, this marker predicts the end-stage CKD [161].
Methylation of the p66Shc promoter in blood is another ESRD biomarker. p66Shc is a stress response protein involved in the oxidative stress and atherosclerosis development. Decreased methylation of the p66Shc promoter in peripheral blood mononuclear cells in patients with ESRD was found to correlate with an increased risk of cardiovascular death [162]. It is assumed that the decrease in the p66Shc promoter methylation can be a result of hyperhomocysteinemia, which often accompanies kidney diseases.
Multiple genomic sites have has been discovered whose methylation levels are associated with ESRD [163-166]. However, there were almost no overlaps between the results of these studies, although three of them used the same technology (Infinium HumanMethylation450 Beadchip) to assess DNA methylation, suggesting the need for comprehensive investigation of these sites in order to identify individual features of their regulation under different conditions.
Some of these regions have been studied in more detail, e.g., the methylenetetrahydrofolate reductase (MTHFR) gene. MTHFR regulates metabolism of methyl groups and homocysteine. Methylation of the MTHFR gene is significantly upregulated in patients with ESRD, which correlates with lower GFR, glycated hemoglobin (HbA1C) content, glycemia, and total and low-density-lipoprotein cholesterol content [165, 167]. Methylation of the MTHFR gene is also associated with hyperhomocysteinemia, which can lead to further glomerular damage and GFR reduction [168].
CKD can cause premature aging of renal tissue, so the use of epigenetic clocks in the assessment of kidney health may be of some scientific interest. The correlation between epigenetic age and biomarkers of renal failure has been shown by several methods [169, 170]. It was found that GFR is associated with the Horvath, Hannum, and Pheno accelerated age scores, MS (mortality risk score based on 10 prominent mortality-related CpG sites), and extrinsic epigenetic age acceleration age/lifespan predictors that can be evaluated by analysis of DNA methylation.
CHANGES IN THE ENDOCRINE SYSTEM AS CKD AND ESRD BIOMARKERS
Hormones play an important role at the endocrine and paracrine levels in the functioning of kidneys as osmoregulatory and detoxifying organs. Normally, kidneys are involved in the excretion of hormones, such as cortisol, aldosterone, sex steroids, thyroid hormones, and catecholamines, and in biodegradation of peptide hormones (e.g., parathyroid hormone, calcitonin, and insulin) [171]. In the course of CKD progression (stages 3-5) [172-174], production, metabolism, and clearance of hormones are disrupted, which causes their accumulation in the blood and more active excretion in the urine. Almost all endocrine axes (from hypothalamus to specific endocrine glands) undergo significant changes during CKD [171, 175].
Changes in the hormone levels in physiological fluids and renal tissue can suggest kidney fibrosis and serve as an indication for changes in the therapeutic strategy in the CKD treatment. Below, we discuss if the hormones controlling the water-salt balance and blood pressure can be used as markers of CKD and ESRD (Table 5).
Renin–angiotensin–aldosterone system. Angiotensin II (AngII) is the principal components of renin–angiotensin–aldosterone system (RAAS) involved in the regulation of vascular tone, blood pressure, and sodium homeostasis [176, 177]. This hormone is a powerful aggravating factor of kidney fibrosis. AngII directly and indirectly stimulates transforming growth factor beta 1 (TGFβ1)-mediated signaling and ECM accumulation upon activation of the profibrotic signaling pathway initiated by activation of AT1 receptors [176, 178, 179]. RAAS includes both circulatory and local components, with intrarenal RAAS being the most developed one, since the kidney has all necessary substrates and enzymes for its functioning.
Intrarenal RAAS plays a special role in the CKD-to-ESRD transition, and its activation is critically important in the renal pathophysiology in CKD patients. Increased renal expression of angiotensinogen (AGT), chymase, and AT1 receptors promotes intrarenal RAAS activation and is associated with kidney damage even after the start of dialysis [180]. Activation of the intrarenal RAAS also occurs in kidney donors without preexisting kidney damage, which increases the risk of developing ESRD by the donor; however, it does not correlate with changes in the blood pressure, eGFR, plasma AngII levels, and albuminuria [181].
Urinary AGT is used as a biomarker of intrarenal RAAS activation in patients with CKD [182, 183], although some researchers believe that the urinary AGT/creatinine ratio is a more informative parameter than the AGT content alone [184, 185]. High urinary AGT levels are associated with a more than 50% decrease in the eGFR in non-dialysis CKD patients, as shown by the Korean Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD) [185]. Similar results on the association of urinary AGT with an adverse renal outcome have been obtained in the studies of patients with autosomal dominant polycystic kidney disease [184] and type 2 diabetes mellitus [184, 186].
Natriuretic peptides. Natriuretic peptides act as RAAS antagonists in the regulation of vascular tone and blood pressure. They protect against hypertension and related pathologies, including kidney damage [187]. Natriuretic peptide family includes atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), C-type natriuretic peptide (CNP), guanylin, and uroguanylin.
Measurements of the plasma concentrations of ANP and BNP can be useful for the risk stratification of patients with ESRD [188, 189]. The plasma levels of ANP and BNP and their N-terminal fragments are increased in patients with CKD, and therefore, have been suggested as prognostic markers of the CKD-to-ESRD transition and fibrosis [189-192]. The main causes for the increased serum concentration of these hormones are hypervolemia, arterial hypertension, and concomitant heart disease, as well as a sharp decrease in or complete absence of renal clearance [188, 193]. High mortality in patients with ESRD is due to a significant risk of developing heart failure associated with a high incidence of left ventricular hypertrophy, various cardiomyopathies, and coronary heart disease [194].
CNP plays an important role in preventing kidney tissue remodeling [195, 196]. Hypoxia and increased release of cytokines and profibrotic growth factors accompanying remodeling stimulate CNP production and secretion [197]. Among all natriuretic peptides, CNP is the first to be degraded by neutral endopeptidase [198], which hinders its detection in physiological fluids. Since the serum content of CNP is very low, it is believed that CNP and products of its degradation should be analyzed in urine rather than in blood [197]. A number of authors consider urinary CNP as an early marker of tubulointerstitial fibrosis. A significant decrease in the urinary CNP was found in the rat UUO model; moreover, this decrease was detected before the emergence of albuminuria and proteinuria and increase in the blood content of creatinine and urea nitrogen [199].
Guanylin is a low-molecular-weight natriuretic peptide produced mainly by intestinal mucosal cells and kidneys [200-202]. Guanylin is associated with various kidney diseases; for example, increased guanylin plasma levels have been observed in patients with CKD [203], glomerulonephritis, and nephrotic syndrome [204]. Uroguanylin, which is similar in structure to guanylin, has been poorly studied in the context of renal pathologies, but its plasma concentration is known to increase in patients with CKD and glomerulonephritis [205]. There are data on the elevated plasma levels of uroguanylin in hemodialysis patients. An increase in the serum concentration of uroguanylin is strongly contributed by the reduced GFR in such patients [206] and amount of table salt consumed by the patients with food [207].
Erythropoietin. Erythropoietin is a glycoprotein synthesized in the kidney. One of its main functions is stimulation of erythropoiesis [208]. Since CKD and ESRD lead to significant disturbances in the erythropoietin synthesis, patients with varying degrees of kidney damage develop anemia [209, 210]. According to the World Health Organization, anemia is defined as a decrease in hemoglobin level to less than 13 g/dl in males and 12 g/dl in females before the menopause. The majority of patients (90%) with the GFR of 25 ml/min are diagnosed with anemia since their hemoglobin level is less than 10 g/dl [211]. Administration of exogenous erythropoietin slows down CKD development, reduces cardiovascular mortality, and increases exercise tolerance [212, 213].
Prolactin. Prolactin is involved in regulation of lactation, reproduction, immunomodulation, and natriuresis [207, 214-217]. A significant elevation of the prolactin blood content is observed in CKD patients and in up to 70% patients on hemodialysis due to the reduced clearance of this hormone [218, 219]. Increasing the frequency of dialysis procedure does not reduce the serum concentration of prolactin [220], unlike with other hormones (for example, dialysis resolves the problem of hyperinsulinemia in patients with CKD-associated insulin resistance [171]). In diagnostics, urine and serum are typically assayed for the so-called macroprolactin, which is a complex of prolactin with IgG with a molecular weight of >100 kDa [221, 222]. According to the published data, hyperprolactinemia in ESRD is an indirect indication for the kidney transplantation.
Kallikrein–kinin system. The kallikrein–kinin system includes polyfunctional blood proteins acting at the endo- and paracrine levels and responsible for the regulation of blood pressure and vasodilation mediated by the nitric oxide release. The most important proteins of this system are kallikrein and bradykinin. A positive correlation between the urinary kallikrein/creatinine ratio and decrease in the eGFR in CKD patients, as well as the urinary content of the inflammatory marker MCP-1, has been found, which makes kallikrein a potential biomarker for assessing the functional state of kidneys [223]. Similar data have been obtained for patients with type 1 diabetes. In these patients, decreased activity of plasma kallikrein was associated with the progression of diabetic nephropathy, the lowest activity of this serine protease being found in patients on hemodialysis. It was suggested that preventing the loss of kallikrein in diabetic nephropathy could preserve the functional activity of kidneys [224]. The promoter of the kallikrein gene KLK1 is characterized by polymorphism, which complicates the identification of gene alleles. However, one of the alleles was found to be more common in ESRD patients than in the control group [225].
PROSPECTS OF ESRD DIAGNOSTICS
Integration of the above biomarkers into everyday diagnostics will allow to attain personalized approach to patients in order to better predict the disease outcome and to select the treatment. The clinical picture can change during the course of CKD depending on the disease itself and personal characteristics of a patient. Hence, we can observe accumulation of different biomarkers in different diseases (Fig. 3).
Some biomarkers have been identified in a particular disease only and therefore, can be used for selective diagnostics of this disease, while other have been detected in two, three, or more major renal disorders (e.g., C3M and MMP-7 were found in all mentioned pathologies). It seems also helpful to use a combination of several biomarkers n for disease diagnostics. For example, C3M and MMP-7 (with eGFR) are the major diagnostic parameters in the assessment of ESRD development. The use of other markers (TNFRs, BMP-7, DcR2, PRO-C6, PRO-C3, CTGF, MCP-1, CXCL16) can narrow the spectrum of diagnosed diseases. Thus, endotrophin and TGF-β are specific for IgA nephropathy, whereas galectin 3 is specific for diabetic nephropathy only. Hormone markers (kallikrein, AGT, guanylin, and uroguanylin) predict ESRD only in patients with diabetic nephropathy or glomerulonephritis.
Epigenetic markers, such as miR-21, miR-93-5p, and miR-92, are not unique for ESRD: miR-146a is specific for IgA nephropathy and glomerulonephritis. The other markers shown in the diagram are also specific for particular diseases. There are some limitations for the application of miRs in clinical practice. For example, there are insufficient data on the correlation between miRs and particular diseases. Inclusion of miRs in the diagram in Fig. 3 is based on currently available scientific data, so in the future, it may turn out that all these miRs should be in the middle of the circles. It might give an impression that all known miRs are only weakly specific for the disorders leading to the CKD development and that the use of miRs alone as biomarkers is insufficient to determine the patient’s condition and to omit eGFR as a diagnostic factor. However, different miRs have their specific characteristics, and their combination could be a powerful diagnostic tool (for example, in a form of test strips used for rapid simultaneous detection of multiple miRs).
CONCLUSION
At present, there are multiple biomarkers associated with CKD transition into ESRD that have been identified in clinical studies and animal models. Some of them are specific to a particular pathology, while others are more versatile. Here, we reported putative inflammatory, fibrosis, hormonal, and epigenetic markers indicative of severe deterioration of renal function and ESRD progression. Their application together with conventional renal failure indicators, such as eGFR, can improve the prognosis of ESRD for immediate initiation of therapy or recommendation for transplantation.
Abbreviations
- AGT:
-
angiotensinogen
- α-SMA:
-
α-smooth muscle actin
- BNP:
-
brain natriuretic peptide
- BMP-7:
-
bone morphogenetic protein 7
- CKD:
-
chronic kidney disease
- CNP:
-
C-type natriuretic peptide
- CTGF:
-
connective tissue growth factor
- CXCL16:
-
C-X-C motif chemokine ligand 16
- DcR2:
-
decoy receptor 2
- ECM:
-
extracellular matrix
- ESRD:
-
end-stage renal disease
- GFR:
-
glomerular filtration rate
- HE4:
-
human epididymal secretory protein E4
- MCP-1:
-
monocyte chemoattractant protein 1
- miRs:
-
microRNAs
- MTHFR:
-
methylenetetrahydrofolate reductase
- RAAS:
-
renin-angiotensin-aldosterone system
- TGFβ1:
-
transforming growth factor beta 1
- UUO:
-
unilateral ureteral obstruction
- PRO-C6:
-
fragment reflecting collagen VI formation
- C1M:
-
fragment of collagen I hydrolysis with MMP
- C3M:
-
fragment reflecting collagen III degradation
- PRO-C3:
-
fragment reflecting collagen III formation
- PINP:
-
procollagen type I N-terminal propeptide
- PIIINP:
-
procollagen type III N-terminal propeptide
References
Schainuck, L. I., Striker, G. E., Cutler, R. E., and Benditt, E. P. (1970) Structural-functional correlations in renal disease. II. The correlations, Hum. Pathol., 1, 631-641, https://doi.org/10.1016/S0046-8177(70)80061-2.
Bohle, A., Mackensen-Haen, S., and von Gise, H. (1987) Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution, Am. J. Nephrol., 7, 421-433, https://doi.org/10.1159/000167514.
Risdon, R. A., Sloper, J. C., and De Wardener, H. E. (1968) Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis, Lancet, 292, 363-366, https://doi.org/10.1016/S0140-6736(68)90589-8.
Hashmi, M. F., Benjamin, O., and Lappin, S. L. (2023) End-Stage Renal Disease, In StatPearls, StatPearls Publishing.
Roufosse, C., Simmonds, N., Clahsen-van Groningen, M., Haas, M., Henriksen, K. J., Horsfield, C., Loupy, A., Mengel, M., Perkowska-Ptasińska, A., Rabant, M., Racusen, L. C., Solez, K., and Becker, J. U. (2018) A 2018 reference guide to the banff classification of renal allograft pathology, Transplantation, 102, 1795-1814, https://doi.org/10.1097/TP.0000000000002366.
Saran, R., Robinson, B., Abbott, K. C., Agodoa, L. Y. C., Bragg-Gresham, J., Balkrishnan, R., Bhave, N., Dietrich, X., Ding, Z., Eggers, P. W., Gaipov, A., Gillen, D., Gipson, D., Gu, H., Guro, P., Haggerty, D., Han, Y., He, K., Herman, W., et al. (2019) US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States, Am. J. Kidney Dis., 73 (3 Suppl 1), A7-A8, https://doi.org/10.1053/j.ajkd.2019.01.001..
Levey, A. S., de Jong, P. E., Coresh, J., El Nahas, M., Astor, B. C., Matsushita, K., Gansevoort, R. T., Kasiske, B. L., and Eckardt, K.-U. (2011) The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report, Kidney Int., 80, 17-28, https://doi.org/10.1038/ki.2010.483.
Acosta-Ochoa, I., Bustamante-Munguira, J., Mendiluce-Herrero, A., Bustamante-Bustamante, J., and Coca-Rojo, A. (2019) Impact on outcomes across KDIGO-2012 AKI criteria according to baseline renal function, J. Clin. Med. Res., 8, https://doi.org/10.3390/jcm8091323.
Uslu, A., Hür, E., Şen, Ç., Şen, S., Akgün, A., Taşlı, F. A., Nart, A., Yilmaz, M., and Töz, H. (2015) To what extent estimated or measured GFR could predict subclinical graft fibrosis: a comparative prospective study with protocol biopsies, Transplant. Int., 28, 575-581, https://doi.org/10.1111/tri.12534.
Bjornstad, P., Karger, A. B., and Maahs, D. M. (2018) Measured GFR in routine clinical practice-the promise of dried blood spots, Adv. Chronic Kidney Dis., 25, 76-83, https://doi.org/10.1053/j.ackd.2017.09.003.
Zsom, L., Zsom, M., Salim, S. A., and Fülöp, T. (2022) Estimated glomerular filtration rate in chronic kidney disease: a critical review of estimate-based predictions of individual outcomes in kidney disease, Toxins, 14, 127, https://doi.org/10.3390/toxins14020127.
Henderson, N. C., Rieder, F., and Wynn, T. A. (2020) Fibrosis: from mechanisms to medicines, Nature, 7835, 555-566, https://doi.org/10.1038/s41586-020-2938-9.
Moeller, M. J., Kramann, R., Lammers, T., Hoppe, B., Latz, E., Ludwig-Portugall, I., Boor, P., Floege, J., Kurts, C., Weiskirchen, R., and Ostendorf, T. (2021) New aspects of kidney fibrosis-from mechanisms of injury to modulation of disease, Front. Med., 8, 814497, https://doi.org/10.3389/fmed.2021.814497.
Ghaderian, S. B., Hayati, F., Shayanpour, S., and Beladi Mousavi, S. S. (2015) Diabetes and end-stage renal disease; a review article on new concepts, J. Renal Injury Prevent., 4, 28-33, https://doi.org/10.12861/jrip.2015.07.
Watanabe, K., Sato, E., Mishima, E., Miyazaki, M., and Tanaka, T. (2022) What’s new in the molecular mechanisms of diabetic kidney disease: recent advances, Int. J. Mol. Sci., 24, 570, https://doi.org/10.3390/ijms24010570.
Lees, J. S., Welsh, C. E., Celis-Morales, C. A., Mackay, D., Lewsey, J., Gray, S. R., Lyall, D. M., Cleland, J. G., Gill, J. M. R., Jhund, P. S., Pell, J., Sattar, N., Welsh, P., and Mark, P. B. (2019) Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease, Nat. Med., 25, 1753-1760, https://doi.org/10.1038/s41591-019-0627-8.
Frąk, W., Kućmierz, J., Szlagor, M., Młynarska, E., Rysz, J., and Franczyk, B. (2022) New insights into molecular mechanisms of chronic kidney disease, Biomedicines, 10, 2846, https://doi.org/10.3390/biomedicines10112846.
Gusev, E., Solomatina, L., Zhuravleva, Y., and Sarapultsev, A. (2021) The Pathogenesis of end-stage renal disease from the standpoint of the theory of general pathological processes of inflammation, Int. J. Mol. Sci., 22, 11453, https://doi.org/10.3390/ijms222111453.
Hewitson, T. D. (2012) Fibrosis in the kidney: is a problem shared a problem halved? Fibrogen. Tissue Rep., 5 (Suppl 1), S14, https://doi.org/10.1186/1755-1536-5-S1-S14.
Barreto, D. V., Barreto, F. C., Liabeuf, S., Temmar, M., Lemke, H.-D., Tribouilloy, C., Choukroun, G., Vanholder, R., Massy, Z. A., and European Uremic Toxin Work Group (EUTox) (2010) Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease, Kidney Int., 77, 550-556, https://doi.org/10.1038/ki.2009.503.
Alicic, R. Z., Johnson, E. J., and Tuttle, K. R. (2018) Inflammatory mechanisms as new biomarkers and therapeutic targets for diabetic kidney disease, Adv. Chronic Kidney Dis., 25, 181-191, https://doi.org/10.1053/j.ackd.2017.12.002.
Fried, L., Solomon, C., Shlipak, M., Seliger, S., Stehman-Breen, C., Bleyer, A. J., Chaves, P., Furberg, C., Kuller, L., and Newman, A. (2004) Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals, J. Am. Soc. Nephrol., 15, 3184-3191, https://doi.org/10.1097/01.ASN.0000146422.45434.35.
Keller, C., Katz, R., Sarnak, M. J., Fried, L. F., Kestenbaum, B., Cushman, M., Shlipak, M. G., and CHS study (2010) Inflammatory biomarkers and decline in kidney function in the elderly: the cardiovascular health study, Nephrol. Dial. Transplant., 25, 119-124, https://doi.org/10.1093/ndt/gfp429.
Mentz, R. J., Kelly, J. P., von Lueder, T. G., Voors, A. A., Lam, C. S. P., Cowie, M. R., Kjeldsen, K., Jankowska, E. A., Atar, D., Butler, J., Fiuzat, M., Zannad, F., Pitt, B., and O’Connor, C. M. (2014) Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction, J. Am. Coll. Cardiol., 64, 2281-2293, https://doi.org/10.1016/j.jacc.2014.08.036.
Pouleur, A.-C. (2015) Which biomarkers do clinicians need for diagnosis and management of heart failure with reduced ejection fraction? Clin. Chim. Acta, 443, 9-16, https://doi.org/10.1016/j.cca.2014.10.046.
Feng, Y.-M., Thijs, L., Zhang, Z.-Y., Yang, W.-Y., Huang, Q.-F., Wei, F.-F., Kuznetsova, T., Jennings, A.-M., Delles, C., Lennox, R., Verhamme, P., Dominiczak, A., and Staessen, J. A. (2017) Glomerular function in relation to circulating adhesion molecules and inflammation markers in a general population, Nephrol. Dial. Transplant., 33, 426-435, https://doi.org/10.1093/ndt/gfx256.
Chiang, C.-K., Hsu, S.-P., Pai, M.-F., Peng, Y.-S., Ho, T.-I., Liu, S.-H., Hung, K.-Y., Tsai, T.-J., and Hsieh, B.-S. (2005) Plasma interleukin-18 levels in chronic renal failure and continuous ambulatory peritoneal dialysis, Blood Purif., 23, 144-148, https://doi.org/10.1159/000083620.
Khanijou, V., Zafari, N., Coughlan, M. T., MacIsaac, R. J., and Ekinci, E. I. (2022) Review of potential biomarkers of inflammation and kidney injury in diabetic kidney disease, Diab. Metab. Res. Rev., 38, e3556, https://doi.org/10.1002/dmrr.3556.
Oh, Y. J., An, J. N., Kim, C. T., Yang, S. H., Lee, H., Kim, D. K., Joo, K. W., Paik, J. H., Kang, S.-W., Park, J. T., Lim, C. S., Kim, Y. S., and Lee, J. P. (2015) Circulating tumor necrosis factor α receptors predict the outcomes of human IgA nephropathy: a prospective cohort study, PLoS One, 10, e0132826, https://doi.org/10.1371/journal.pone.0132826.
Niewczas, M. A., Pavkov, M. E., Skupien, J., Smiles, A., Md Dom, Z. I., Wilson, J. M., Park, J., Nair, V., Schlafly, A., Saulnier, P.-J., Satake, E., Simeone, C. A., Shah, H., Qiu, C., Looker, H. C., Fiorina, P., Ware, C. F., Sun, J. K., Doria, A., et al. (2019) A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes, Nat. Med., 25, 805-813, https://doi.org/10.1038/s41591-019-0415-5.
Khan, F., Kapoor, S., Rana, J., and Khan, S. (2022) Evaluation of Inflammatory markers in different stages of chronic renal disease, Asian J. Med. Sci., 13, 100-107, https://doi.org/10.3126/ajms.v13i5.40454.
Fathi, F., Atapour, A., Eskandari, N., Keyhanmehr, N., Hafezi, H., Mohammadi, S., and Motedayyen, H. (2019) Regulatory T-cells and their impacts on cytokine profile of end-stage renal disease patients suffering from systemic lupus erythematosus, Int. J. Immunopathol. Pharmacol., 33, https://doi.org/10.1177/2058738419863238.
Abraham, R., Durkee, M. S., Ai, J., Veselits, M., Casella, G., Asano, Y., Chang, A., Ko, K., Oshinsky, C., Peninger, E., Giger, M. L., and Clark, M. R. (2022) Specific in situ inflammatory states associate with progression to renal failure in lupus nephritis, J. Clin. Invest., 132, e155350, https://doi.org/10.1172/JCI155350.
Lioulios, G., Fylaktou, A., Xochelli, A., Sampani, E., Tsouchnikas, I., Giamalis, P., Daikidou, D.-V., Nikolaidou, V., Papagianni, A., Theodorou, I., and Stangou, M. (2022) Clustering of end stage renal disease patients by dimensionality reduction algorithms according to lymphocyte senescence markers, Front. Immunol., 13, 841031, https://doi.org/10.3389/fimmu.2022.841031.
Turkmen, K., Guney, I., Yerlikaya, F. H., and Tonbul, H. Z. (2012) The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients, Renal Failure, 34, 155-159, https://doi.org/10.3109/0886022X.2011.641514.
Turkmen, K., Erdur, F. M., Ozcicek, F., Ozcicek, A., Akbas, E. M., Ozbicer, A., Demirtas, L., Turk, S., and Tonbul, H. Z. (2013) Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients, Hemodial. Int., 17, 391-396, https://doi.org/10.1111/hdi.12040.
Ahbap, E., Sakaci, T., Kara, E., Sahutoglu, T., Koc, Y., Basturk, T., Sevinc, M., Akgol, C., Kayalar, A. O., Ucar, Z. A., Bayraktar, F., and Unsal, A. (2016) Neutrophil-to-lymphocyte ratio and platelet-tolymphocyte ratio in evaluation of inflammation in end-stage renal disease, Clin. Nephrol., 85, 199-208, https://doi.org/10.5414/CN108584.
Catabay, C., Obi, Y., Streja, E., Soohoo, M., Park, C., Rhee, C. M., Kovesdy, C. P., Hamano, T., and Kalantar-Zadeh, K. (2017) Lymphocyte cell ratios and mortality among incident hemodialysis patients, Am. J. Nephrol., 46, 408-416, https://doi.org/10.1159/000484177.
Ouellet, G., Malhotra, R., Penne, E. L., Usvya, L., Levin, N. W., and Kotanko, P. (2016) Neutrophil-lymphocyte ratio as a novel predictor of survival in chronic hemodialysis patients, Clin. Nephrol., 85, 191-198, https://doi.org/10.5414/CN108745.
Li, P., Xia, C., Liu, P., Peng, Z., Huang, H., Wu, J., and He, Z. (2020) Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in evaluation of inflammation in non-dialysis patients with end-stage renal disease (ESRD), BMC Nephrology, 21, 511, https://doi.org/10.1186/s12882-020-02174-0.
Keane, T. J., Horejs, C.-M., and Stevens, M. M. (2018) Scarring vs. functional healing: matrix-based strategies to regulate tissue repair, Adv. Drug Deliv. Rev., 129, 407-419, https://doi.org/10.1016/j.addr.2018.02.002.
GBD Chronic Kidney Disease Collaboration (2020) Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017, Lancet, 395, 709-733, https://doi.org/10.1016/S0140-6736(20)30045-3.
Djudjaj, S., and Boor, P. (2019) Cellular and molecular mechanisms of kidney fibrosis, Mol. Aspects Med., 65, 16-36, https://doi.org/10.1016/j.mam.2018.06.002.
Genovese, F., Manresa, A. A., Leeming, D. J., Karsdal, M. A., and Boor, P. (2014) The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Rep., 7, 4, https://doi.org/10.1186/1755-1536-7-4.
Buchtler, S., Grill, A., Hofmarksrichter, S., Stöckert, P., Schiechl-Brachner, G., Rodriguez Gomez, M., Neumayer, S., Schmidbauer, K., Talke, Y., Klinkhammer, B. M., Boor, P., Medvinsky, A., Renner, K., Castrop, H., and Mack, M. (2018) Cellular origin and functional relevance of collagen I production in the kidney, J. Am. Soc. Nephrol., 29, 1859-1873, https://doi.org/10.1681/ASN.2018020138.
Sharma, A. K., Mauer, S. M., Kim, Y., and Michael, A. F. (1993) Interstitial fibrosis in obstructive nephropathy, Kidney Int., 44, 774-788, https://doi.org/10.1038/ki.1993.312.
Mason, R. M., and Wahab, N. A. (2003) Extracellular matrix metabolism in diabetic nephropathy, J. Am. Soc. Nephrol., 14, 1358-1373, https://doi.org/10.1097/01.ASN.0000065640.77499.D7.
Vleming, L. J., Baelde, J. J., Westendorp, R. G., Daha, M. R., van Es, L. A., and Bruijn, J. A. (1995) Progression of chronic renal disease in humans is associated with the deposition of basement membrane components and decorin in the interstitial extracellular matrix, Clin. Nephrol., 44, 211-219.
Boor, P., Konieczny, A., Villa, L., Kunter, U., van Roeyen, C. R. C., LaRochelle, W. J., Smithson, G., Arrol, S., Ostendorf, T., and Floege, J. (2007) PDGF-D inhibition by CR002 ameliorates tubulointerstitial fibrosis following experimental glomerulonephritis, Nephrol. Dial. Transplant., 22, 1323-1331, https://doi.org/10.1093/ndt/gfl691.
Boor, P., Celec, P., Behuliak, M., Grancic, P., Kebis, A., Kukan, M., Pronayová, N., Liptaj, T., Ostendorf, T., and Sebeková, K. (2009) Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats, Metab. Clin. Exp., 58, 1669-1677, https://doi.org/10.1016/j.metabol.2009.05.025.
Hewitson, T. D., Smith, E. R., and Samuel, C. S. (2014) Qualitative and quantitative analysis of fibrosis in the kidney, Nephrology, 19, 721-726, https://doi.org/10.1111/nep.12321.
Rangan, G. K. and Tesch, G. H. (2007) Quantification of renal pathology by image analysis, Nephrology, 12, 553-558, https://doi.org/10.1111/j.1440-1797.2007.00855.x.
Bertram, J. F. (2001) Counting in the kidney, Kidney Int., 59, 792-796, https://doi.org/10.1046/j.1523-1755.2001.059002792.x.
Ricard-Blum, S., and Ruggiero, F. (2005) The collagen superfamily: from the extracellular matrix to the cell membrane, Patholog. Biol., 53, 430-442, https://doi.org/10.1016/j.patbio.2004.12.024.
Ignat’eva, N. Y., Danilov, N. A., Averkiev, S. V., Obrezkova, M. V., Lunin, V. V., and Sobol’, E. N. (2007) Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues, J. Anal. Chem., 62, 51-57, https://doi.org/10.1134/S106193480701011X.
Genovese, F., Rasmussen, D. G. K., Karsdal, M. A., Jesky, M., Ferro, C., Fenton, A., and Cockwell, P. (2021) Imbalanced turnover of collagen type III is associated with disease progression and mortality in high-risk chronic kidney disease patients, Clin. Kidney J., 14, 593-601, https://doi.org/10.1093/ckj/sfz174.
Poulsen, C. G., Rasmussen, D. G. K., Genovese, F., Hansen, T. W., Nielsen, S. H., Reinhard, H., von Scholten, B. J., Jacobsen, P. K., Parving, H.-H., Karsdal, M. A., Rossing, P., and Frimodt-Møller, M. (2023) Marker for kidney fibrosis is associated with inflammation and deterioration of kidney function in people with type 2 diabetes and microalbuminuria, PLoS One, 18, e0283296, https://doi.org/10.1371/journal.pone.0283296.
Mavrogeorgis, E., Mischak, H., Latosinska, A., Vlahou, A., Schanstra, J. P., Siwy, J., Jankowski, V., Beige, J., and Jankowski, J. (2021) Collagen-derived peptides in CKD: a link to fibrosis, Toxins, 14, 10, https://doi.org/10.3390/toxins14010010.
Morita, M., Uchigata, Y., Hanai, K., Ogawa, Y., and Iwamoto, Y. (2011) Association of urinary type IV collagen with GFR decline in young patients with type 1 diabetes, Am. J. Kidney Dis., 58, 915-920, https://doi.org/10.1053/j.ajkd.2011.04.019.
Klimontov, V. V., Eremenko, N. V., Myakina, N. E., and Fazullina, O. N. (2015) Cystatin C and collagen type IV in diagnostics of chronic kidney disease in type 2 diabetic patients, Diabetes Mellitus, 18, 87-93, https://doi.org/10.14341/dm2015187-93.
Pilemann-Lyberg, S., Rasmussen, D. G. K., Hansen, T. W., Tofte, N., Winther, S. A., Holm Nielsen, S., Theilade, S., Karsdal, M. A., Genovese, F., and Rossing, P. (2019) Markers of collagen formation and degradation reflect renal function and predict adverse outcomes in patients with type 1 diabetes, Diabetes Care, 42, 1760-1768, https://doi.org/10.2337/dc18-2599.
Fenton, A., Jesky, M. D., Ferro, C. J., Sørensen, J., Karsdal, M. A., Cockwell, P., and Genovese, F. (2017) Serum endotrophin, a type VI collagen cleavage product, is associated with increased mortality in chronic kidney disease, PLoS One, 12, e0175200, https://doi.org/10.1371/journal.pone.0175200.
Rasmussen, D. G. K., Fenton, A., Jesky, M., Ferro, C., Boor, P., Tepel, M., Karsdal, M. A., Genovese, F., and Cockwell, P. (2017) Urinary endotrophin predicts disease progression in patients with chronic kidney disease, Sci. Rep., 7, 17328, https://doi.org/10.1038/s41598-017-17470-3.
Den Hoedt, C. H., van Gelder, M. K., Grooteman, M. P., Nubé, M. J., Blankestijn, P. J., Goldschmeding, R., Kok, R. J., Bots, M. L., van den Dorpel, M. A., and Gerritsen, K. G. F. (2019) Connective tissue growth factor is related to all-cause mortality in hemodialysis patients and is lowered by on-line hemodiafiltration: results from the convective transport study, Toxins, 11, 268, https://doi.org/10.3390/toxins11050268.
Soylemezoglu, O., Wild, G., Dalley, A. J., MacNeil, S., Milford-Ward, A., Brown, C. B., and el Nahas, A. M. (1997) Urinary and serum type III collagen: markers of renal fibrosis, Nephrol. Dial. Transplant., 12, 1883-1889, https://doi.org/10.1093/ndt/12.9.1883.
Teppo, A.-M., Törnroth, T., Honkanen, E., and Grönhagen-Riska, C. (2003) Urinary amino-terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients, Transplantation, 75, 2113-2119, https://doi.org/10.1097/01.TP.0000066809.60389.48.
Ghoul, B. E., Squalli, T., Servais, A., Elie, C., Meas-Yedid, V., Trivint, C., Vanmassenhove, J., Grünfeld, J.-P., Olivo-Marin, J.-C., Thervet, E., Noël, L.-H., Prié, D., and Fakhouri, F. (2010) Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist, Clin. J. Am. Soc. Nephrol., 5, 205-210, https://doi.org/10.2215/CJN.06610909.
Predictive Value of PIIINP Urinary for the Development of Chronic Renal Failure in Patients with Cystic Fibrosis After Lung Transplantation (MUCO-IRC) (2012, April 6) ClinicalTrials.gov, URL: https://clinicaltrials.gov/ct2/show/NCT01572194?cond=NCT01572194anddraw=2andrank=1.
Ix, J. H., Katz, R., Bansal, N., Foster, M., Weiner, D. E., Tracy, R., Jotwani, V., Hughes-Austin, J., McKay, D., Gabbai, F., Hsu, C.-Y., Bostom, A., Levey, A. S., and Shlipak, M. G. (2017) Urine fibrosis markers and risk of allograft failure in kidney transplant recipients: a case-cohort ancillary study of the FAVORIT trial, Am. J. Kidney Dis., 69, 410-419, https://doi.org/10.1053/j.ajkd.2016.10.019.
Papasotiriou, M., Genovese, F., Klinkhammer, B. M., Kunter, U., Nielsen, S. H., Karsdal, M. A., Floege, J., and Boor, P. (2015) Serum and urine markers of collagen degradation reflect renal fibrosis in experimental kidney diseases, Nephrol. Dial. Transplant., 30, 1112-1121, https://doi.org/10.1093/ndt/gfv063.
Genovese, F., Akhgar, A., Lim, S. S., Farris, A. B., Battle, M., Cobb, J., Sinibaldi, D., Karsdal, M. A., and White, W. I. (2021) Collagen type III and VI remodeling biomarkers are associated with kidney fibrosis in Lupus nephritis, Kidney360, 2, 1473-1481, https://doi.org/10.34067/KID.0001132021.
Lee, Y. H., Kim, K. P., Park, S.-H., Kim, D.-J., Kim, Y.-G., Moon, J.-Y., Jung, S.-W., Kim, J. S., Jeong, K.-H., Lee, S.-Y., Yang, D.-H., Lim, S.-J., Woo, J.-T., Rhee, S. Y., Chon, S., Choi, H.-Y., Park, H.-C., Jo, Y.-I., Yi, J.-H., et al. (2021) Urinary chemokine C-X-C motif ligand 16 and endostatin as predictors of tubulointerstitial fibrosis in patients with advanced diabetic kidney disease, Nephrol. Dial. Transplant., 36, 295-305, https://doi.org/10.1093/ndt/gfz168.
Lin, C. H. S., Chen, J., Ziman, B., Marshall, S., Maizel, J., and Goligorsky, M. S. (2014) Endostatin and kidney fibrosis in aging: a case for antagonistic pleiotropy? Am. J. Physiol. Heart Circ. Physiol., 306, H1692-H1699, https://doi.org/10.1152/ajpheart.00064.2014.
Caterino, M., Zacchia, M., Costanzo, M., Bruno, G., Arcaniolo, D., Trepiccione, F., Siciliano, R. A., Mazzeo, M. F., Ruoppolo, M., and Capasso, G. (2018) Urine proteomics revealed a significant correlation between urine-fibronectin abundance and estimated-GFR decline in patients with Bardet–Biedl syndrome, Kidney Blood Press. Res., 43, 389-405, https://doi.org/10.1159/000488096.
Cao, Y. H., Lv, L. L., Zhang, X., Hu, H., Ding, L. H., Yin, D., Zhang, Y. Z., Ni, H. F., Chen, P. S., and Liu, B. C. (2015) Urinary vimentin mRNA as a potential novel biomarker of renal fibrosis, Am. J. Physiol. Renal Physiol., 309, F514-F522, https://doi.org/10.1152/ajprenal.00449.2014.
Eddy, A. A. (1996) Molecular insights into renal interstitial fibrosis, J. Am. Soc. Nephrol., 7, 2495-2508, https://doi.org/10.1681/ASN.V7122495.
Sakairi, T., Hiromura, K., Yamashita, S., Takeuchi, S., Tomioka, M., Ideura, H., Maeshima, A., Kaneko, Y., Kuroiwa, T., Nangaku, M., Takeuchi, T., and Nojima, Y. (2007) Nestin expression in the kidney with an obstructed ureter, Kidney Int., 72, 307-318, https://doi.org/10.1038/sj.ki.5002277.
Hugo, C., Shankland, S. J., Pichler, R. H., Couser, W. G., and Johnson, R. J. (1998) Thrombospondin 1 precedes and predicts the development of tubulointerstitial fibrosis in glomerular disease in the rat, Kidney Int., 53, 302-311, https://doi.org/10.1046/j.1523-1755.1998.00774.x.
Wang, Z., Divanyan, A., Jourd’heuil, F. L., Goldman, R. D., Ridge, K. M., Jourd’heuil, D., and Lopez-Soler, R. I. (2018) Vimentin expression is required for the development of EMT-related renal fibrosis following unilateral ureteral obstruction in mice, Am. J. Physiol. Renal Physiol., 315, F769-F780, https://doi.org/10.1152/ajprenal.00340.2017.
Duffield, J. S. (2014) Cellular and molecular mechanisms in kidney fibrosis, J. Clin. Invest., 124, 2299-2306, https://doi.org/10.1172/JCI72267.
Lepreux, S., and Desmoulière, A. (2015) Human liver myofibroblasts during development and diseases with a focus on portal (myo)fibroblasts, Front. Physiol., 6, 173, https://doi.org/10.3389/fphys.2015.00173.
Hinz, B. (2016) Myofibroblasts, Exp. Eye Res., 142, 56-70, https://doi.org/10.1016/j.exer.2015.07.009.
Sun, K.-H., Chang, Y., Reed, N. I., and Sheppard, D. (2016) α-Smooth muscle actin is an inconsistent marker of fibroblasts responsible for force-dependent TGFβ activation or collagen production across multiple models of organ fibrosis, Am. J. Physiol. Lung Cell. Mol. Physiol., 310, L824-L836, https://doi.org/10.1152/ajplung.00350.2015.
Bochaton-Piallat, M.-L., Gabbiani, G., and Hinz, B. (2016) The myofibroblast in wound Bohle, A., Mackensen-Haen, S., and von Gise, H. (1987) Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution, Am. J. Nephrol., 7, 421-433, https://doi.org/10.1159/000167514.
Timsit, M. O., Gadet, R., Ben Abdennebi, H., Codas, R., Petruzzo, P., and Badet, L. (2008) Renal ischemic preconditioning improves recovery of kidney function and decreases alpha-smooth muscle actin expression in a rat model, J. Urology, 180, 388-391, https://doi.org/10.1016/j.juro.2008.02.043.
Singh, S. P., Tao, S., Fields, T. A., Webb, S., Harris, R. C., and Rao, R. (2015) Glycogen synthase kinase-3 inhibition attenuates fibroblast activation and development of fibrosis following renal ischemia-reperfusion in mice, Dis. Models Mech., 8, 931-940, https://doi.org/10.1242/dmm.020511.
Zhang, A., Wang, H., Wang, B., Yuan, Y., Klein, J. D., and Wang, X. H. (2019) Exogenous miR-26a suppresses muscle wasting and renal fibrosis in obstructive kidney disease, FASEB J., 33, 13590-13601, https://doi.org/10.1096/fj.201900884R.
Wong, M. G., and Pollock, C. A. (2014) Biomarkers in kidney fibrosis: are they useful? Kidney Int. Suppl., 4, 79-83, https://doi.org/10.1038/kisup.2014.15.
Wong, M. G., Perkovic, V., Woodward, M., Chalmers, J., Li, Q., Hillis, G. S., Yaghobian Azari, D., Jun, M., Poulter, N., Hamet, P., Williams, B., Neal, B., Mancia, G., Cooper, M., and Pollock, C. A. (2013) Circulating bone morphogenetic protein-7 and transforming growth factor-β1 are better predictors of renal end points in patients with type 2 diabetes mellitus, Kidney Int., 83, 278-284, https://doi.org/10.1038/ki.2012.383.
Ito, Y., Aten, J., Bende, R. J., Oemar, B. S., Rabelink, T. J., Weening, J. J., and Goldschmeding, R. (1998) Expression of connective tissue growth factor in human renal fibrosis, Kidney Int., 53, 853-861, https://doi.org/10.1111/j.1523-1755.1998.00820.x.
Phanish, M. K., Winn, S. K., and Dockrell, M. E. C. (2010) Connective tissue growth factor-(CTGF, CCN2) – a marker, mediator and therapeutic target for renal fibrosis, Nephron Exp. Nephrol., 114, e83-e92, https://doi.org/10.1159/000262316.
Nguyen, T. Q., Tarnow, L., Jorsal, A., Oliver, N., Roestenberg, P., Ito, Y., Parving, H.-H., Rossing, P., van Nieuwenhoven, F. A., and Goldschmeding, R. (2008) Plasma connective tissue growth factor is an independent predictor of end-stage renal disease and mortality in type 1 diabetic nephropathy, Diabetes Care, 31, 1177-1182, https://doi.org/10.2337/dc07-2469.
Chen, J., Hu, W., Xiao, F., Lin, L., Chen, K., Wang, L., Wang, X., and He, Y. (2019) DCR2, a cellular senescent molecule, is a novel marker for assessing tubulointerstitial fibrosis in patients with immunoglobulin a nephropathy, Kidney Blood Press. Res., 44, 1063-1074, https://doi.org/10.1159/000502233.
Ju, W., Nair, V., Smith, S., Zhu, L., Shedden, K., Song, P. X. K., Mariani, L. H., Eichinger, F. H., Berthier, C. C., Randolph, A., Lai, J. Y.-C., Zhou, Y., Hawkins, J. J., Bitzer, M., Sampson, M. G., Thier, M., Solier, C., Duran-Pacheco, G. C., Duchateau-Nguyen, G. (2015) Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker, Sci. Translat. Med., 7, 316ra193, https://doi.org/10.1126/scitranslmed.aac7071.
Nowak, G., and Schnellmann, R. G. (1995) Integrative effects of EGF on metabolism and proliferation in renal proximal tubular cells, Am. J. Physiol., 269 (5 Pt 1), C1317-C1325, https://doi.org/10.1152/ajpcell.1995.269.5.C1317.
Isaka, Y. (2016) Epidermal growth factor as a prognostic biomarker in chronic kidney diseases, Ann. Translat. Med., 4 (Suppl 1), S62, https://doi.org/10.21037/atm.2016.10.64.
Kim, J. E., Han, D., Jeong, J. S., Moon, J. J., Moon, H. K., Lee, S., Kim, Y. C., Yoo, K. D., Lee, J. W., Kim, D. K., Kwon, Y. J., Kim, Y. S., and Yang, S. H. (2021) Multisample mass spectrometry-based approach for discovering injury markers in chronic kidney disease, Mol. Cell. Proteomics, 20, 100037, https://doi.org/10.1074/mcp.RA120.002159.
Tang, W. H. W., Shrestha, K., Shao, Z., Borowski, A. G., Troughton, R. W., Thomas, J. D., and Klein, A. L. (2011) Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival, Am. J. Cardiol., 108, 385-390, https://doi.org/10.1016/j.amjcard.2011.03.056.
Sotomayor, C. G., Te Velde-Keyzer, C. A., Diepstra, A., van Londen, M., Pol, R. A., Post, A., Gans, R. O. B., Nolte, I. M., Slart, R. H. J. A., de Borst, M. H., Berger, S. P., Rodrigo, R., Navis, G. J., de Boer, R. A., and Bakker, S. J. L. (2021) Galectin-3 and risk of late graft failure in kidney transplant recipients: a 10-year prospective cohort study, Transplantation, 105, 1106-1115, https://doi.org/10.1097/TP.0000000000003359.
O’Seaghdha, C. M., Hwang, S.-J., Ho, J. E., Vasan, R. S., Levy, D., and Fox, C. S. (2013) Elevated galectin-3 precedes the development of CKD, J. Am. Soc. Nephrol., 24, 1470-1477, https://doi.org/10.1681/ASN.2012090909.
Ou, S.-M., Tsai, M.-T., Chen, H.-Y., Li, F.-A., Tseng, W.-C., Lee, K.-H., Chang, F.-P., Lin, Y.-P., Yang, R.-B., and Tarng, D.-C. (2021) Identification of galectin-3 as potential biomarkers for renal fibrosis by RNA-sequencing and clinicopathologic findings of kidney biopsy, Front. Med., 8, 748225, https://doi.org/10.3389/fmed.2021.748225.
Ou, S.-M., Tsai, M.-T., Chen, H.-Y., Li, F.-A., Lee, K.-H., Tseng, W.-C., Chang, F.-P., Lin, Y.-P., Yang, R.-B., and Tarng, D.-C. (2022) Urinary galectin-3 as a novel biomarker for the prediction of renal fibrosis and kidney disease progression, Biomedicines, 10, 585, https://doi.org/10.3390/biomedicines10030585.
LeBleu, V. S., Teng, Y., O’Connell, J. T., Charytan, D., Müller, G. A., Müller, C. A., Sugimoto, H., and Kalluri, R. (2013) Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis, Nat. Med., 19, 227-231, https://doi.org/10.1038/nm.2989.
Yuan, T., and Li, Y. (2017) Human epididymis protein 4 as a potential biomarker of chronic kidney disease in female patients with normal ovarian function, Lab. Med., 48, 238-243, https://doi.org/10.1093/labmed/lmx036.
Amer, H., Lieske, J. C., Rule, A. D., Kremers, W. K., Larson, T. S., Franco Palacios, C. R., Stegall, M. D., and Cosio, F. G. (2013) Urine high and low molecular weight proteins one-year post-kidney transplant: relationship to histology and graft survival, Am. J. Transplant., 13, 676-684, https://doi.org/10.1111/ajt.12044.
Nadkarni, G. N., Rao, V., Ismail-Beigi, F., Fonseca, V. A., Shah, S. V., Simonson, M. S., Cantley, L., Devarajan, P., Parikh, C. R., and Coca, S. G. (2016) Association of urinary biomarkers of inflammation, injury, and fibrosis with renal function decline: the ACCORD trial, Clin. J. Am. Soc. Nephrol., 11, 1343-1352, https://doi.org/10.2215/CJN.12051115.
Park, M., Katz, R., Shlipak, M. G., Weiner, D., Tracy, R., Jotwani, V., Hughes-Austin, J., Gabbai, F., Hsu, C. Y., Pfeffer, M., Bansal, N., Bostom, A., Gutierrez, O., Sarnak, M., Levey, A., and Ix, J. H. (2017) Urinary markers of fibrosis and risk of cardiovascular events and death in kidney transplant recipients: the FAVORIT trial, Am. J. Transplant., 17, 2640-2649, https://doi.org/10.1111/ajt.14284.
Glassock, R. J. (2016) Urinary chemoattractant protein 1: a new biomarker of renal fibrosis, Am. J. Nephrol., 43, 451-453, https://doi.org/10.1159/000446864.
Ihara, K., Skupien, J., Kobayashi, H., Md Dom, Z. I., Wilson, J. M., O’Neil, K., Badger, H. S., Bowsman, L. M., Satake, E., Breyer, M. D., Duffin, K. L., and Krolewski, A. S. (2020) Profibrotic circulating proteins and risk of early progressive renal decline in patients with type 2 diabetes with and without albuminuria, Diabetes Care, 43, 2760-2767, https://doi.org/10.2337/dc20-0630.
Zhou, D., Tian, Y., Sun, L., Zhou, L., Xiao, L., Tan, R. J., Tian, J., Fu, H., Hou, F. F., and Liu, Y. (2017) Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis, J. Am. Soc. Nephrol., 28, 598-611, https://doi.org/10.1681/ASN.2016030354.
Pallet, N., Chauvet, S., Chassé, J.-F., Vincent, M., Avillach, P., Levi, C., Meas-Yedid, V., Olivo-Marin, J.-C., Nga-Matsogo, D., Beaune, P., Thervet, E., and Karras, A. (2014) Urinary retinol binding protein is a marker of the extent of interstitial kidney fibrosis, PLoS One, 9, e84708, https://doi.org/10.1371/journal.pone.0084708.
Honkanen, E., Teppo, A. M., Törnroth, T., Groop, P. H., and Grönhagen-Riska, C. (1997) Urinary transforming growth factor-beta 1 in membranous glomerulonephritis, Nephrol. Dial. Transplant., 12, 2562-2568, https://doi.org/10.1093/ndt/12.12.2562.
Susianti, H., Handono, K., Gunawan, A., Mintaroem, K., Purnomo, B. B., and Kalim, H. (2015) Transforming growth factor β1 is better than α smooth muscle actin for the prediction of renal fibrosis in patients with nephritic lupus, Biomarkers Genomic Med., 7, 25-30, https://doi.org/10.1016/j.bgm.2014.08.010.
Murakami, K., Takemura, T., Hino, S., and Yoshioka, K. (1997) Urinary transforming growth factor-beta in patients with glomerular diseases, Pediatric Nephrol., 11, 334-336, https://doi.org/10.1007/s004670050289.
Harris, S., Coupes, B. M., Roberts, S. A., Roberts, I. S. D., Short, C. D., and Brenchley, P. E. C. (2007) TGF-beta1 in chronic allograft nephropathy following renal transplantation, J. Nephrol., 20, 177-185.
Chan, J., Svensson, M., Tannæs, T. M., Waldum-Grevbo, B., Jenssen, T., and Eide, I. A. (2022) Associations of serum uromodulin and urinary epidermal growth factor with measured glomerular filtration rate and interstitial fibrosis in kidney transplantation, Am. J. Nephrol., 53, 108-117, https://doi.org/10.1159/000521757.
Hussain, S., Habib, A., Hussain, M. S., and Najmi, A. K. (2020) Potential biomarkers for early detection of diabetic kidney disease, Diabetes Res. Clin. Pract., 161, 108082, https://doi.org/10.1016/j.diabres.2020.108082.
Lin, Z., Gong, Q., Zhou, Z., Zhang, W., Liao, S., Liu, Y., Yan, X., Pan, X., Lin, S., and Li, X. (2011) Increased plasma CXCL16 levels in patients with chronic kidney diseases, Eur. J. Clin. Invest., 41, 836-845, https://doi.org/10.1111/j.1365-2362.2011.02473.x.
Unal, H. U., Kurt, Y. G., Gok, M., Cetinkaya, H., Karaman, M., Eyileten, T., Vural, A., Oguz, Y., and Yilmaz, M. I. (2014) The importance of serum CXCL-16 levels in patients with grade III-V chronic kidney disease, Turkish Nephrol. Dial. Transplant., 23, 234-239, https://doi.org/10.5262/tndt.2014.1003.10.
Ruge, T., Carlsson, A. C., Larsson, T. E., Carrero, J.-J., Larsson, A., Lind, L., and Ärnlöv, J. (2014) Endostatin level is associated with kidney injury in the elderly: findings from two community-based cohorts, Am. J. Nephrol., 40, 417-424, https://doi.org/10.1159/000369076.
Chen, J., Hamm, L. L., Kleinpeter, M. A., Husserl, F., Khan, I. E., Chen, C.-S., Liu, Y., Mills, K. T., He, C., Rifai, N., Simon, E. E., and He, J. (2012) Elevated plasma levels of endostatin are associated with chronic kidney disease, Am. J. Nephrology, 35, 335-340, https://doi.org/10.1159/000336109.
Chu, C., Hasan, A. A., Gaballa, M. M. S., Zeng, S., Xiong, Y., Elitok, S., Krämer, B. K., and Hocher, B. (2020) Endostatin is an independent risk factor of graft loss after kidney transplant, Am. J. Nephrology, 51, 373-380, https://doi.org/10.1159/000507824.
Carlsson, A. C., Östgren, C. J., Länne, T., Larsson, A., Nystrom, F. H., and Ärnlöv, J. (2016) The association between endostatin and kidney disease and mortality in patients with type 2 diabetes, Diab. Metab., 42, 351-357, https://doi.org/10.1016/j.diabet.2016.03.006.
Gregg, L. P., Tio, M. C., Li, X., Adams-Huet, B., de Lemos, J. A., and Hedayati, S. S. (2018) Association of monocyte chemoattractant protein-1 with death and atherosclerotic events in chronic kidney disease, Am. J. Nephrol., 47, 395-405, https://doi.org/10.1159/000488806.
Domingos, M. A. M., Moreira, S. R., Gomez, L., Goulart, A., Lotufo, P. A., Benseñor, I., and Titan, S. (2016) Urinary retinol-binding protein: relationship to renal function and cardiovascular risk factors in chronic kidney disease, PLoS One, 11, e0162782, https://doi.org/10.1371/journal.pone.0162782.
Gerritsen, K. G., Abrahams, A. C., Peters, H. P., Nguyen, T. Q., Koeners, M. P., den Hoedt, C. H., Dendooven, A., van den Dorpel, M. A., Blankestijn, P. J., Wetzels, J. F., Joles, J. A., Goldschmeding, R., and Kok, R. J. (2012) Effect of GFR on plasma N-terminal connective tissue growth factor (CTGF) concentrations, Am. J. Kidney Dis., 59, 619-627, https://doi.org/10.1053/j.ajkd.2011.12.019.
Wan, J., Wang, Y., Cai, G., Liang, J., Yue, C., Wang, F., Song, J., Wang, J., Liu, M., Luo, J., and Li, L. (2016) Elevated serum concentrations of HE4 as a novel biomarker of disease severity and renal fibrosis in kidney disease, Oncotarget, 7, 67748-67759, https://doi.org/10.18632/oncotarget.11682.
Shen, X., Cheng, J., Yu, G., Li, X., Li, H., and Chen, J. (2021) Urine β2-Microglobulin and Retinol-Binding Protein and Renal Disease Progression in IgA nephropathy, Front. Med., 8, 792782, https://doi.org/10.3389/fmed.2021.792782.
Tachaudomdach, C., Kantachuvesiri, S., Changsirikulchai, S., Wimolluck, S., Pinpradap, K., and Kitiyakara, C. (2012) Connective tissue growth factor gene expression and decline in renal function in lupus nephritis, Exp. Ther. Med., 3, 713-718, https://doi.org/10.3892/etm.2012.473.
Steubl, D., Buzkova, P., Garimella, P. S., Ix, J. H., Devarajan, P., Bennett, M. R., Chaves, P. H. M., Shlipak, M. G., Bansal, N., and Sarnak, M. J. (2019) Association of serum uromodulin with ESKD and kidney function decline in the elderly: the cardiovascular health study, Am. J. Kidney Dis., 74, 501-509, https://doi.org/10.1053/j.ajkd.2019.02.024.
Badid, C., Desmouliere, A., Babici, D., Hadj-Aissa, A., McGregor, B., Lefrancois, N., Touraine, J. L., and Laville, M. (2002) Interstitial expression of alpha-SMA: an early marker of chronic renal allograft dysfunction, Nephrol. Dial. Transplant., 17, 1993-1998, https://doi.org/10.1093/ndt/17.11.1993.
Kashtan, C., Schachter, A., Klickstein, L., Liu, X., Jennings, L., and Finkel, N. (2022) Urinary monocyte chemoattractant protein-1 in patients with Alport syndrome, Kidney Int. Rep., 7, 1112-1114, https://doi.org/10.1016/j.ekir.2022.01.1052.
Yang, X., Ou, J., Zhang, H., Xu, X., Zhu, L., Li, Q., Li, J., Xie, D., Sun, J., Zha, Y., Li, Y., Tian, J., Liu, Y., and Hou, F. F. (2020) Urinary matrix metalloproteinase 7 and prediction of IgA Nephropathy progression, Am. J. Kidney Dis., 75, 384-393, https://doi.org/10.1053/j.ajkd.2019.07.018.
Kato, M., and Natarajan, R. (2019) Epigenetics and epigenomics in diabetic kidney disease and metabolic memory, Nat. Rev. Nephrol., 15, 327-345, https://doi.org/10.1038/s41581-019-0135-6.
Ding, H., Zhang, L., Yang, Q., Zhang, X., and Li, X. (2021) Epigenetics in kidney diseases, Adv. Clin. Chem., 104, 233-297, https://doi.org/10.1016/bs.acc.2020.09.005.
Tampe, B., and Zeisberg, M. (2014) Contribution of genetics and epigenetics to progression of kidney fibrosis, Nephrol. Dial. Transplant., 29, iv72-iv79, https://doi.org/10.1093/ndt/gft025.
Zhao, H., Pan, S., Duan, J., Liu, F., Li, G., Liu, D., and Liu, Z. (2021) Integrative analysis of mA regulator-mediated RNA methylation modification patterns and immune characteristics in lupus nephritis, Front. Cell Dev. Biol., 9, 724837, https://doi.org/10.3389/fcell.2021.724837.
Chou, Y.-H., Pan, S.-Y., Shao, Y.-H., Shih, H.-M., Wei, S.-Y., Lai, C.-F., Chiang, W.-C., Schrimpf, C., Yang, K.-C., Lai, L.-C., Chen, Y.-M., Chu, T.-S., and Lin, S.-L. (2020) Methylation in pericytes after acute injury promotes chronic kidney disease, J. Clin. Investig., 130, 4845-4857, https://doi.org/10.1172/JCI135773.
Jiang, M., Bai, M., Lei, J., Xie, Y., Xu, S., Jia, Z., and Zhang, A. (2020) Mitochondrial dysfunction and the AKI-to-CKD transition, Am. J. Physiol. Renal Physiol., 319, F1105-F1116, https://doi.org/10.1152/ajprenal.00285.2020.
Tanemoto, F., Nangaku, M., and Mimura, I. (2022) Epigenetic memory contributing to the pathogenesis of AKI-to-CKD transition, Front. Mol. Biosci., 9, 1003227, https://doi.org/10.3389/fmolb.2022.1003227.
Nangaku, M., Hirakawa, Y., Mimura, I., Inagi, R., and Tanaka, T. (2017) Epigenetic changes in the acute kidney injury-to-chronic kidney disease transition, Nephron, 137, 256-259, https://doi.org/10.1159/000476078.
Fan, Y., Chen, H., Huang, Z., Zheng, H., and Zhou, J. (2020) Emerging role of miRNAs in renal fibrosis, RNA Biol., 17, 1-12, https://doi.org/10.1080/15476286.2019.1667215.
Glowacki, F., Savary, G., Gnemmi, V., Buob, D., Van der Hauwaert, C., Lo-Guidice, J.-M., Bouyé, S., Hazzan, M., Pottier, N., Perrais, M., Aubert, S., and Cauffiez, C. (2013) Increased circulating miR-21 levels are associated with kidney fibrosis, PLoS One, 8, e58014, https://doi.org/10.1371/journal.pone.0058014.
Gniewkiewicz, M. S., Paszkowska, I., Gozdowska, J., Czerwinska, K., Sadowska-Jakubowicz, A., Deborska-Materkowska, D., Perkowska-Ptasinska, A., Kosieradzki, M., and Durlik, M. (2020) Urinary MicroRNA-21-5p as potential biomarker of interstitial fibrosis and tubular atrophy (IFTA) in kidney transplant recipients, Diagnostics, 10, 113, https://doi.org/10.3390/diagnostics10020113.
Chen, C., Lu, C., Qian, Y., Li, H., Tan, Y., Cai, L., and Weng, H. (2017) Urinary miR-21 as a potential biomarker of hypertensive kidney injury and fibrosis, Sci. Rep., 7, 17737, https://doi.org/10.1038/s41598-017-18175-3.
Szeto, C.-C., Ching-Ha, K. B., Ka-Bik, L., Mac-Moune, L. F., Cheung-Lung, C. P., Gang, W., Kai-Ming, C., and Kam-Tao, L. P. (2012) Micro-RNA expression in the urinary sediment of patients with chronic kidney diseases, Disease Markers, 33, 137-144, https://doi.org/10.1155/2012/842764.
Pezzolesi, M. G., Satake, E., McDonnell, K. P., Major, M., Smiles, A. M., and Krolewski, A. S. (2015) Circulating TGF-β1-regulated miRNAs and the risk of rapid progression to ESRD in type 1 diabetes, Diabetes, 64, 3285-3293, https://doi.org/10.2337/db15-0116.
Lopez-Anton, M., Lambie, M., Lopez-Cabrera, M., Schmitt, C. P., Ruiz-Carpio, V., Bartosova, M., Schaefer, B., Davies, S., Stone, T., Jenkins, R., Taylor, P. R., Topley, N., Bowen, T., and Fraser, D. (2017) miR-21 promotes fibrogenesis in peritoneal dialysis, Am. J. Pathol., 187, 1537-1550, https://doi.org/10.1016/j.ajpath.2017.03.007.
Chau, B. N., Xin, C., Hartner, J., Ren, S., Castano, A. P., Linn, G., Li, J., Tran, P. T., Kaimal, V., Huang, X., Chang, A. N., Li, S., Kalra, A., Grafals, M., Portilla, D., MacKenna, D. A., Orkin, S. H., and Duffield, J. S. (2012) MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways, Sci. Translat. Med., 4, 121ra18, https://doi.org/10.1126/scitranslmed.3003205.
Lai, J. Y., Luo, J., O’Connor, C., Jing, X., Nair, V., Ju, W., Randolph, A., Ben-Dov, I. Z., Matar, R. N., Briskin, D., Zavadil, J., Nelson, R. G., Tuschl, T., Brosius, F. C., Kretzler, M., and Bitzer, M. (2015) MicroRNA-21 in glomerular injury, J. Am. Soc. Nephrol., 26, 805-816, https://doi.org/10.1681/ASN.2013121274.
Shang, F., Wang, S.-C., Hsu, C.-Y., Miao, Y., Martin, M., Yin, Y., Wu, C.-C., Wang, Y.-T., Wu, G., Chien, S., Huang, H.-D., Tarng, D.-C., Shiu, Y.-T., Cheung, A. K., Huang, P.-H., Chen, Z., and Shyy, J. Y.-J. (2017) MicroRNA-92a mediates endothelial dysfunction in CKD, J. Am. Soc. Nephrol., 28, 3251-3261, https://doi.org/10.1681/ASN.2016111215.
Henique, C., Bollée, G., Loyer, X., Grahammer, F., Dhaun, N., Camus, M., Vernerey, J., Guyonnet, L., Gaillard, F., Lazareth, H., Meyer, C., Bensaada, I., Legrès, L., Satoh, T., Akira, S., Bruneval, P., Dimmeler, S., Tedgui, A., Karras, A., et al. (2017) Genetic and pharmacological inhibition of microRNA-92a maintains podocyte cell cycle quiescence and limits crescentic glomerulonephritis, Nat. Commun., 8, 1829, https://doi.org/10.1038/s41467-017-01885-7.
Ulbing, M., Kirsch, A. H., Leber, B., Lemesch, S., Münzker, J., Schweighofer, N., Hofer, D., Trummer, O., Rosenkranz, A. R., Müller, H., Eller, K., Stadlbauer, V., and Obermayer-Pietsch, B. (2017) MicroRNAs 223-3p and 93-5p in patients with chronic kidney disease before and after renal transplantation, Bone, 95, 115-123, https://doi.org/10.1016/j.bone.2016.11.016.
Han, Q., Zhang, Y., Jiao, T., Li, Q., Ding, X., Zhang, D., Cai, G., and Zhu, H. (2021) Urinary sediment microRNAs can be used as potential noninvasive biomarkers for diagnosis, reflecting the severity and prognosis of diabetic nephropathy, Nutrit. Diabetes, 11, 24, https://doi.org/10.1038/s41387-021-00166-z.
Rivoli, L., Vliegenthart, A. D. B., de Potter, C. M. J., van Bragt, J. J. M. H., Tzoumas, N., Gallacher, P., Farrah, T. E., Dhaun, N., and Dear, J. W. (2017) The effect of renal dysfunction and haemodialysis on circulating liver specific miR-122, Br. J. Clin. Pharmacol., 83, 584-592, https://doi.org/10.1111/bcp.13136.
Chen, N. X., Kiattisunthorn, K., O’Neill, K. D., Chen, X., Moorthi, R. N., Gattone, V. H., 2nd, Allen, M. R., and Moe, S. M. (2013) Decreased microRNA is involved in the vascular remodeling abnormalities in chronic kidney disease (CKD), PLoS One, 8, e64558, https://doi.org/10.1371/journal.pone.0064558.
Wang, H., Peng, W., Shen, X., Huang, Y., Ouyang, X., and Dai, Y. (2012) Circulating levels of inflammation-associated miR-155 and endothelial-enriched miR-126 in patients with end-stage renal disease, Brazil. J. Med. Biol. Res., 45, 1308-1314, https://doi.org/10.1590/S0100-879X2012007500165.
Abdelsalam, L., Ibrahim, A. A., Shalaby, A., Osman, N., Hashad, A., Badawy, D., Elghobary, H., and Amer, E. (2019) Expression of miRNAs-122, -192 and -499 in end stage renal disease associated with acute myocardial infarction, Arch. Med. Sci., 15, 1247-1253, https://doi.org/10.5114/aoms.2019.87095.
Jiang, Z.-H., Tang, Y.-Z., Song, H.-N., Yang, M., Li, B., and Ni, C.-L. (2020) miRNA-342 suppresses renal interstitial fibrosis in diabetic nephropathy by targeting SOX6, Int. J. Mol. Med., 45, 45-52, https://doi.org/10.3892/ijmm.2019.4388.
Fawzy, M. S., Abu AlSel, B. T., Al Ageeli, E., Al-Qahtani, S. A., Abdel-Daim, M. M., and Toraih, E. A. (2020) Long non-coding RNA MALAT1 and microRNA-499a expression profiles in diabetic ESRD patients undergoing dialysis: a preliminary cross-sectional analysis, Arch. Physiol. Biochem., 126, 172-182, https://doi.org/10.1080/13813455.2018.1499119.
Onishi, A., Sugiyama, H., Kitagawa, M., Yamanari, T., Tanaka, K., Ogawa-Akiyama, A., Kano, Y., Mise, K., Tanabe, K., Morinaga, H., Kinomura, M., Uchida, H. A., and Wada, J. (2019) Urine 5MedC, a marker of DNA methylation, in the progression of chronic kidney disease, Dis. Markers, 2019, 5432453, https://doi.org/10.1155/2019/5432453.
Geisel, J., Schorr, H., Heine, G. H., Bodis, M., Hübner, U., Knapp, J.-P., and Herrmann, W. (2007) Decreased p66Shc promoter methylation in patients with end-stage renal disease, Clin. Chem. Lab. Med., 45, 1764-1770, https://doi.org/10.1515/CCLM.2007.357.
Wing, M. R., Devaney, J. M., Joffe, M. M., Xie, D., Feldman, H. I., Dominic, E. A., Guzman, N. J., Ramezani, A., Susztak, K., Herman, J. G., Cope, L., Harmon, B., Kwabi-Addo, B., Gordish-Dressman, H., Go, A. S., He, J., Lash, J. P., Kusek, J. W., Raj, D. S., and Chronic Renal Insufficiency Cohort (CRIC) Study (2014) DNA methylation profile associated with rapid decline in kidney function: findings from the CRIC study, Nephrol. Dial. Transplant., 29, 864-872, https://doi.org/10.1093/ndt/gft537.
Chu, A. Y., Tin, A., Schlosser, P., Ko, Y.-A., Qiu, C., Yao, C., Joehanes, R., Grams, M. E., Liang, L., Gluck, C. A., Liu, C., Coresh, J., Hwang, S.-J., Levy, D., Boerwinkle, E., Pankow, J. S., Yang, Q., Fornage, M., Fox, C. S., et al. (2017) Epigenome-wide association studies identify DNA methylation associated with kidney function, Nat. Commun., 8, 1286, https://doi.org/10.1038/s41467-017-01297-7.
Sapienza, C., Lee, J., Powell, J., Erinle, O., Yafai, F., Reichert, J., Siraj, E. S., and Madaio, M. (2011) DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy, Epigenetics, 6, 20-28, https://doi.org/10.4161/epi.6.1.13362.
Qiu, C., Hanson, R. L., Fufaa, G., Kobes, S., Gluck, C., Huang, J., Chen, Y., Raj, D., Nelson, R. G., Knowler, W. C., and Susztak, K. (2018) Cytosine methylation predicts renal function decline in American Indians, Kidney Int., 93, 1417-1431, https://doi.org/10.1016/j.kint.2018.01.036.
Ghattas, M., El-Shaarawy, F., Mesbah, N., and Abo-Elmatty, D. (2014) DNA methylation status of the methylenetetrahydrofolate reductase gene promoter in peripheral blood of end-stage renal disease patients, Mol. Biol. Rep., 41, 683-688, https://doi.org/10.1007/s11033-013-2906-7.
Li, N., Chen, Y.-F., and Zou, A.-P. (2002) Implications of hyperhomocysteinemia in glomerular sclerosis in hypertension, Hypertension, 39 (2 Pt 2), 443-448, https://doi.org/10.1161/hy02t2.102992.
Yusipov, I., Kondakova, E., Kalyakulina, A., Krivonosov, M., Lobanova, N., Bacalini, M. G., Franceschi, C., Vedunova, M., and Ivanchenko, M. (2022) Accelerated epigenetic aging and inflammatory/immunological profile (ipAGE) in patients with chronic kidney disease, GeroScience, 44, 817-834, https://doi.org/10.1007/s11357-022-00540-4.
Matías-García, P. R., Ward-Caviness, C. K., Raffield, L. M., Gao, X., Zhang, Y., Wilson, R., Gào, X., Nano, J., Bostom, A., Colicino, E., Correa, A., Coull, B., Eaton, C., Hou, L., Just, A. C., Kunze, S., Lange, L., Lange, E., Lin, X., et al. (2021) DNAm-based signatures of accelerated aging and mortality in blood are associated with low renal function, Clin. Epigenet., 13, 121, https://doi.org/10.1186/s13148-021-01082-w.
Niemczyk, S., Niemczyk, L., and Romejko-Ciepielewska, K. (2012) Basic endocrinological disorders in chronic renal failure, Endokrynol. Polska, 63, 250-257.
Panizo, S., Martínez-Arias, L., Alonso-Montes, C., Cannata, P., Martín-Carro, B., Fernández-Martín, J. L., Naves-Díaz, M., Carrillo-López, N., and Cannata-Andía, J. B. (2021) Fibrosis in chronic kidney disease: pathogenesis and consequences, Int. J. Mol. Sci., 22, 408, https://doi.org/10.3390/ijms22010408.
Liu, Y. (2006) Renal fibrosis: new insights into the pathogenesis and therapeutics, Kidney Int., 69, 213-217, https://doi.org/10.1038/sj.ki.5000054.
Cho, M. H. (2010) Renal fibrosis, Korean J. Pediatrics, 53, 735-740, https://doi.org/10.3345/kjp.2010.53.7.735.
Banaei, S., and Rezagholizadeh, L. (2019) The role of hormones in renal disease and ischemia-reperfusion injury, Iranian J. Basic Med. Sci., 22, 469.
AlQudah, M., Hale, T. M., and Czubryt, M. P. (2020) Targeting the renin-angiotensin-aldosterone system in fibrosis, Matrix Biol., 91-92, 92-108, https://doi.org/10.1016/j.matbio.2020.04.005.
Carey, R. M., Wang, Z. Q., and Siragy, H. M. (2000) Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function, Hypertension, 35, 155-163, https://doi.org/10.1161/01.HYP.35.1.155.
Mezzano, S. A., Ruiz-Ortega, M., and Egido, J. (2001) Angiotensin II and renal fibrosis, Hypertension, 38, 635-638, https://doi.org/10.1161/hy09t1.094234.
Balakumar, P., Sambathkumar, R., Mahadevan, N., Muhsinah, A. B., Alsayari, A., Venkateswaramurthy, N., and Jagadeesh, G. (2019) A potential role of the renin-angiotensin-aldosterone system in epithelial-to-mesenchymal transition-induced renal abnormalities: Mechanisms and therapeutic implications, Pharmacol. Res., 146, 104314, https://doi.org/10.1016/j.phrs.2019.104314.
Ohashi, N., Isobe, S., Ishigaki, S., Suzuki, T., Ono, M., Fujikura, T., Tsuji, T., Kato, A., Ozono, S., and Yasuda, H. (2017) Intrarenal renin-angiotensin system activity is augmented after initiation of dialysis, Hypertens. Res., 40, 364-370, https://doi.org/10.1038/hr.2016.143.
Ohashi, N., Isobe, S., Matsuyama, T., Ishigaki, S., Suzuki, T., Tsuji, T., Otsuka, A., Kato, A., Miyake, H., and Yasuda, H. (2019) The intrarenal renin-angiotensin system is activated immediately after kidney donation in kidney transplant donors, Internal Med., 58, 643-648, https://doi.org/10.2169/internalmedicine.1756-18.
Reams, G., Villarreal, D., Wu, Z., and Bauer, J. H. (1994) Urinary angiotensin II: a marker of renal tissue activity? Nephron, 67, 450-458, https://doi.org/10.1159/000188215.
Yamamoto, T., Nakagawa, T., Suzuki, H., Ohashi, N., Fukasawa, H., Fujigaki, Y., Kato, A., Nakamura, Y., Suzuki, F., and Hishida, A. (2007) Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease, J. Am. Soc. Nephrol., 18, 1558-1565, https://doi.org/10.1681/ASN.2006060554.
Park, H. C., Kim, J., Cho, A., Kim, D. H., Lee, Y. K., Ryu, H., Kim, H., Oh, K. H., Oh, Y. K., Hwang, Y. H., Lee, K. B., Kim, S. W., Kim, Y. H., Lee, J., Ahn, C., and KNOW-CKD Investigators Group (2020) Urinary angiotensinogen in addition to imaging classification in the prediction of renal outcome in autosomal dominant polycystic kidney disease, J. Korean Med. Sci., 35, e165, https://doi.org/10.3346/jkms.2020.35.e165.
Suh, S. H., Oh, T. R., Choi, H. S., Yang, E. M., Kim, C. S., Bae, E. H., Ma, S. K., Oh, K.-H., Jung, J. Y., Hyun, Y. Y., and Kim, S. W. (2022) Urinary angiotensinogen and progression of chronic kidney disease: results from KNOW-CKD study, Biomolecules, 12, 1280, https://doi.org/10.3390/biom12091280.
Lee, M. J., Kim, S. S., Kim, I. J., Song, S. H., Kim, E. H., Seo, J. Y., et al. (2017) Changes in urinary angiotensinogen associated with deterioration of kidney function in patients with type 2 diabetes mellitus, J. Korean Med. Sci., 32, 782-788, https://doi.org/10.3346/jkms.2017.32.5.782.
Choi, M. R., and Fernández, B. E. (2021) Protective renal effects of atrial natriuretic peptide: where are we now? Front. Physiol., 12, 680213, https://doi.org/10.3389/fphys.2021.680213.
Khalifeh, N., Haider, D., and Hörl, W. H. (2009) Natriuretic peptides in chronic kidney disease and during renal replacement therapy: an update, J. Invest. Med., 57, 33-39, https://doi.org/10.2310/JIM.0b013e318194f44b.
Wang, A. Y.-M., and Lai, K.-N. (2008) Use of cardiac biomarkers in end-stage renal disease, J. Am. Soc. Nephrol., 19, 1643-1652, https://doi.org/10.1681/ASN.2008010012.
Spanaus, K.-S., Kronenberg, F., Ritz, E., Schlapbach, R., Fliser, D., Hersberger, M., Kollerits, B., König, P., von Eckardstein, A., and Mild-to-Moderate Kidney Disease Study Group (2007) B-type natriuretic peptide concentrations predict the progression of nondiabetic chronic kidney disease: the mild-to-moderate kidney disease study, Clin. Chem., 53, 1264-1272, https://doi.org/10.1373/clinchem.2006.083170.
Dieplinger, B., Mueller, T., Kollerits, B., Struck, J., Ritz, E., von Eckardstein, A., Haltmayer, M., Kronenberg, F., and MMKD Study Group (2009) Pro-A-type natriuretic peptide and pro-adrenomedullin predict progression of chronic kidney disease: the MMKD study, Kidney Int., 75, 408-414, https://doi.org/10.1038/ki.2008.560.
Yandle, T. G., Espiner, E. A., Gary Nicholls, M., and Duff, H. (1986) Radioimmunoassay and characterization of atrial natriuretic peptide in human plasma, J. Clin. Endocrinol. Metab., 63, 72-79, https://doi.org/10.1210/jcem-63-1-72.
Safley, D. M., Awad, A., Sullivan, R. A., Sandberg, K. R., Mourad, I., Boulware, M., Merhi, W., and McCullough, P. A. (2005) Changes in B-type natriuretic peptide levels in hemodialysis and the effect of depressed left ventricular function, Adv. Chronic Kidney Dis., 12, 117-124, https://doi.org/10.1053/j.ackd.2004.11.002.
Wahl, H. G., Graf, S., Renz, H., and Fassbinder, W. (2004) Elimination of the cardiac natriuretic peptides B-type natriuretic peptide (BNP) and N-terminal proBNP by hemodialysis, Clin. Chem., 50, 1071-1074, https://doi.org/10.1373/clinchem.2003.030692.
Cataliotti, A., Giordano, M., De Pascale, E., Giordano, G., Castellino, P., Jougasaki, M., Costello, L. C., Boerrigter, G., Tsuruda, T., Belluardo, P., Lee, S.-C., Huntley, B., Sandberg, S., Malatino, L. S., and Burnett, J. C., Jr. (2002) CNP production in the kidney and effects of protein intake restriction in nephrotic syndrome, Am. J. Physiol. Renal Physiol., 283, F464-F472, https://doi.org/10.1152/ajprenal.00372.2001.
Hu, P., Zhang, X. C., Kong, H. B., Xia, X., Hu, B., and Qin, Y. H. (2015) Exogenous C-type natriuretic peptide infusion ameliorates unilateral ureteral obstruction-induced tubulointerstitial fibrosis in rats, Lab. Invest., 95, 263-272, https://doi.org/10.1038/labinvest.2014.149.
Zakeri, R., Burnett, J. C., Jr, and Sangaralingham, S. J. (2015) Urinary C-type natriuretic peptide: an emerging biomarker for heart failure and renal remodeling, Clin. Chim. Acta, 443, 108-113, https://doi.org/10.1016/j.cca.2014.12.009.
Kenny, A. J., Bourne, A., and Ingram, J. (1993) Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11, Biochem. J., 291, 83-88, https://doi.org/10.1042/bj2910083.
Hu, P., Wang, J., Hu, B., Lu, L., Xuan, Q., and Qin, Y. H. (2012) Increased urinary C-type natriuretic peptide excretion may be an early marker of renal tubulointerstitial fibrosis, Peptides, 37, 98-105, https://doi.org/10.1016/j.peptides.2012.06.009.
Carrithers, S. L., Hill, M. J., Johnson, B. R., O’Hara, S. M., Jackson, B. A., Ott, C. E., Lorenz, J., Mann, E. A., Giannella, R. A., Forte, L. R., and Greenberg, R. N. (1999) Renal effects of uroguanylin and guanylin in vivo, Brazil. J. Med. Biol. Res., 32, 1337-1344, https://doi.org/10.1590/S0100-879X1999001100003.
Sindić, A., and Schlatter, E. (2005) Mechanisms of actions of guanylin peptides in the kidney, Pflugers Arch., 450, 283-291, https://doi.org/10.1007/s00424-005-1464-9.
Sindić, A., and Schlatter, E. (2007) Renal electrolyte effects of guanylin and uroguanylin, Curr. Opin. Nephrol. Hypertens., 16, 10-15, https://doi.org/10.1097/MNH.0b013e328011cb4a.
Nakazato, M., Yamaguchi, H., Shiomi, K., Date, Y., Fujimoto, S., Kangawa, K., Matsuo, H., and Matsukura, S. (1994) Identification of 10-kDa proguanylin as a major guanylin molecule in human intestine and plasma and its increase in renal insufficiency, Biochem. Biophys. Res. Commun., 205, 1966-1975, https://doi.org/10.1006/bbrc.1994.2901.
Kinoshita, H., Fujimoto, S., Fukae, H., Yokota, N., Hisanaga, S., Nakazato, M., and Eto, T. (1999) Plasma and urine levels of uroguanylin, a new natriuretic peptide, in nephrotic syndrome, Nephron, 81, 160-164, https://doi.org/10.1159/000045272.
Nakazato, M., Yamaguchi, H., Kinoshita, H., Kangawa, K., Matsuo, H., Chino, N., and Matsukura, S. (1996) Identification of biologically active and inactive human uroguanylins in plasma and urine and their increases in renal insufficiency, Biochem. Biophys. Res. Commun., 220, 586-593, https://doi.org/10.1006/bbrc.1996.0447.
Fukae, H., Kinoshita, H., Fujimoto, S., Nakazato, M., and Eto, T. (2000) Plasma concentration of uroguanylin in patients on maintenance dialysis therapy, Nephron, 84, 206-210, https://doi.org/10.1159/000045578.
Kinoshita, H., Fujimoto, S., Nakazato, M., Yokota, N., Date, Y., Yamaguchi, H., Hisanaga, S., and Eto, T. (1997) Urine and plasma levels of uroguanylin and its molecular forms in renal diseases, Kidney Int., 52, 1028-1034, https://doi.org/10.1038/ki.1997.424.
Sasaki, R., Masuda, S., and Nagao, M. (2000) Erythropoietin: multiple physiological functions and regulation of biosynthesis, Biosci. Biotechnol. Biochem., 64, 1775-1793, https://doi.org/10.1271/bbb.64.1775.
Hayat, A. (2009) Erythropoietin friend or foe in chronic kidney disease anemia: an analysis of randomized controlled trials, observational studies and meta-analyses, Br. J. Med. Practit., 2, 12-20.
Sacks, D., Baxter, B., Campbell, B. C. V., Carpenter, J. S., Cognard, C., Dippel, D., Eesa, M., Fischer, U., Hausegger, K., Hirsch, J. A., Shazam Hussain, M., Jansen, O., Jayaraman, M. V., Khalessi, A. A., Kluck, B. W., Lavine, S., Meyers, P. M., Ramee, S., Rüfenacht, D. A., et al. (2018) Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke, Int. J. Stroke, 13, 612-632, https://doi.org/10.1016/j.jvir.2017.11.026.
Lankhorst, C. E., and Wish, J. B. (2010) Anemia in renal disease: diagnosis and management, Blood Rev., 24, 39-47, https://doi.org/10.1016/j.blre.2009.09.001.
Provatopoulou, S. T., and Ziroyiannis, P. N. (2011) Clinical use of erythropoietin in chronic kidney disease: outcomes and future prospects, Hippokratia, 15, 109-115.
Ghasemi, F., Abdi, A., Salari, N., Tohidi, M. R., and Faraji, A. (2019) Comparing the effects of intravenous and subcutaneous Erythropoietin on blood indices in hemodialysis patients, Sci. Rep., 9, 2284, https://doi.org/10.1038/s41598-018-38193-z.
Ben-Jonathan, N., LaPensee, C. R., and LaPensee, E. W. (2008) What can we learn from rodents about prolactin in humans? Endocr. Rev., 29, 1-41, https://doi.org/10.1210/er.2007-0017.
Freeman, M. E., Kanyicska, B., Lerant, A., and Nagy, G. (2000) Prolactin: structure, function, and regulation of secretion, Physiol. Rev., 80, 1523-1631, https://doi.org/10.1152/physrev.2000.80.4.1523.
Devi, Y. S., Sangeeta Devi, Y., and Halperin, J. (2014) Reproductive actions of prolactin mediated through short and long receptor isoforms, Mol. Cell. Endocrinol., 382, 400-410, https://doi.org/10.1016/j.mce.2013.09.016.
Takada, M., and Hokari, S. (2007) Prolactin increases Na+ transport across adult bullfrog skin via stimulation of both ENaC and Na+/K+-pump, Gen. Comp. Endocrinol., 151, 325-331, https://doi.org/10.1016/j.ygcen.2007.01.040.
Sievertsen, G. D., Lim, V. S., Nakawatase, C., and Frohman, L. A. (1980) Metabolic clearance and secretion rates of human prolactin in normal subjects and in patients with chronic renal failure, J. Clin. Endocrinol. Metab., 50, 846-852, https://doi.org/10.1210/jcem-50-5-846.
Dourado, M., Cavalcanti, F., Vilar, L., and Cantilino, A. (2020) Relationship between prolactin, chronic kidney disease, and cardiovascular risk, Int. J. Endocrinol., 2020, 9524839, https://doi.org/10.1155/2020/9524839.
Lo, J. C., Beck, G. J., Kaysen, G. A., Chan, C. T., Kliger, A. S., Rocco, M. V., and Chertow, G. M. for the FHN Study (2017) Hyperprolactinemia in end-stage renal disease and effects of frequent hemodialysis, Hemodial. Int., 2, 190-196, https://doi.org/10.1111/hdi.12489.
Shimatsu, A., and Hattori, N. (2012) Macroprolactinemia: diagnostic, clinical, and pathogenic significance, Clin. Dev. Immunol., 2012, 167132, https://doi.org/10.1155/2012/167132.
Huang, W., and Molitch, M. E. (2021) Prolactin and other pituitary disorders in kidney disease, Semin. Nephrol., 41, 156-167, https://doi.org/10.1016/j.semnephrol.2021.03.010.
Chiang, W.-C., Lin, S.-L., Chen, Y.-M., Wu, K.-D., and Tsai, T.-J. (2008) Urinary kallikrein excretion is related to renal function change and inflammatory status in chronic kidney disease patients receiving angiotensin II receptor blocker treatment, Nephrology, 13, 198-203, https://doi.org/10.1111/j.1440-1797.2008.00933.x.
Härma, M.-A., Dahlström, E. H., Sandholm, N., Forsblom, C., Groop, P.-H., Lehto, M., and FinnDiane Study Group (2020) Decreased plasma kallikrein activity is associated with reduced kidney function in individuals with type 1 diabetes, Diabetologia, 63, 1349-1354, https://doi.org/10.1007/s00125-020-05144-1.
Yu, H., Song, Q., Freedman, B. I., Chao, J., Chao, L., Rich, S. S., and Bowden, D. W. (2002) Association of the tissue kallikrein gene promoter with ESRD and hypertension, Kidney Int., 61, 1030-1039, https://doi.org/10.1046/j.1523-1755.2002.00198.x.
Study of Urinary Angiotensinogen as a Marker to Warn the Deterioration of Renal Function in CKD Patients Early. (n.d.) ClinicalTrials.gov. Retrieved September 2009, URL: https://clinicaltrials.gov/ct2/show/NCT01118494?cond=NCT01118494anddraw=2andrank=1.
Funding
This work was supported by the Russian Science Foundation (project 22-74-00058).
Author information
Authors and Affiliations
Contributions
All authors wrote the manuscript; E.Y.P. edited the text.
Corresponding author
Ethics declarations
The authors declare no conflict of interest. This article does not contain description of studies involving human participants or animals performed by any of the authors.
Rights and permissions
Open access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yakupova, E.I., Abramicheva, P.A., Bocharnikov, A.D. et al. Biomarkers of the End-Stage Renal Disease Progression: Beyond the GFR. Biochemistry Moscow 88, 1622–1644 (2023). https://doi.org/10.1134/S0006297923100164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923100164