Abstract
Chromatin spatial organization in the nucleus is essential for the genome functioning and regulation of gene activity. The nuclear lamina and lamina-associated proteins, lamins, play a key role in this process. Lamin dysfunction leads to the decompaction and transcriptional activation of heterochromatin, which is associated with the premature aging syndrome. In many cell types, telomeres are located at the nuclear periphery, where their replication and stability are ensured by the nuclear lamina. Moreover, diseases associated with defects in lamins and telomeres have similar manifestations and resemble physiological aging. Understanding molecular changes associated with aging at the organismal level is especially important. In this study, we compared the effects caused by the mutation in lamin B and physiological aging in the germline of the model organism Drosophila melanogaster. We have shown that the impaired localization of lamin B leads to the heterochromatin decompaction and transcriptional activation of some transposable elements and telomeric repeats. Both DNA damage and activation of homologous recombination in the telomeres were observed in the germ cells of lamin B mutants. The instability of repeat-enriched heterochromatin can be directly related to the genome destabilization, germ cell death, and sterility observed in lamin B mutants. Similar processes were observed in Drosophila germline in the course of physiological aging, which indicates a close link between the maintenance of the heterochromatin stability at the nuclear periphery and mechanisms of aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
At the molecular level, cellular aging is associated with the heterochromatin decompaction, transcriptional activation of normally silent genomic repeats, and disruption of telomere homeostasis [1]. Similar processes are characteristic of cells with dysfunctional lamin proteins associated with the nuclear envelope. This observation has led to the concept of laminopathy as a key process of cell aging [2]. The processes occurring at the nuclear periphery ensure the genome stability; the most important of them are the maintenance of heterochromatin enriched with repeats of various nature and, therefore, prone to ectopic recombination.
The association of heterochromatin with the nuclear lamina maintains the compact structure of the heterochromatin and provides its recombination stability [3]. An important role of lamin proteins in the aging process and oncogenesis has been confirmed by the experimental data obtained mainly in cellular models [4]. The aging of senescent cells is accompanied by changes in the chromatin structure, redistribution of lamina-associated domains (LADs), activation of transcription of heterochromatic repeats, and telomere dysfunction [5, 6]. The same phenomena are caused by impairments in the functioning of lamins [7, 8]. However, the causal relationship between such complex processes as aging and laminopathy is still poorly understood.
Lamins are structural proteins associated with the nuclear envelope. They are involved in various cellular processes, such as the maintenance of the nuclear architecture, DNA replication and repair, and regulation of transcription [9]. There are two main types of lamins: lamins A/C and lamins B. Lamin A is located both on the nuclear envelope and in the nucleoplasm, while the B-type lamins are found exclusively at the nuclear periphery. Both types of lamins are involved in the attachment of LADs to the nuclear envelope, which determines the nuclear architecture [10]. Experiments on the knockdown of both types of lamins in Drosophila cells revealed global changes in the chromatin folding, such as euchromatin compaction and heterochromatin relaxation [11]. The dysfunction of lamins leads not only to the structural alterations of the genome, but also to the disturbances in the DNA replication and repair at the nuclear periphery [5, 12-14]. A mutation in the human LMNA gene encoding lamin A that results in the synthesis of truncated lamin protein causes Hutchinson–Gilford progeria (accelerated aging), which is also accompanied by the impaired gene expression [15] and telomere shortening [16]. Lamin B plays a key role in the heterochromatin homeostasis; the disruption of the interactions between lamin B and heterochromatin regions is associated with the cell transition to senescence or cell aging [17]. Telomeres are heterochromatin domains predominantly located near the nuclear envelope in yeast and mammalian cells [18]. Mammalian telomere proteins physically interact with lamins, which provides telomere localization at the nuclear periphery and affects telomere repair, replication, and transcription [2, 18]. Moreover, diseases caused by the defects in lamins and telomeres have similar manifestations and resemble premature aging [19].
Can model organisms with mutations in lamins be used as bona fide models of aging at the organismal level? To address this question, it is necessary to identify the key molecular processes associated with mutations in lamins and to compare them with the processes occurring during physiological aging at the organismal level. This approach will help to find new diagnostic markers of aging and, possibly, to identify the metabolic pathways that can be manipulated to prevent premature aging. The studies in Drosophila melanogaster have shown that the decompaction of heterochromatin and activation of transposable elements in somatic cells occur in both aging tissues and lamin B mutants [20, 21]. Suppression of the transposon activity and proper heterochromatin maintenance increase the lifespan, which is an argument in favor of the retrotransposon theory of aging [22]. Both aging cells and Drosophila lamin mutants are characterized by the DNA damage [20]. Despite the lack of complete understanding of the aging mechanisms, it is obvious that the destabilization of heterochromatin associated with the nuclear periphery is one of the most important signs of aging.
The lamin B null mutants of D. melanogaster are viable, but sterile [23]. This interesting fact indicates an important role of lamin B in the gametogenesis. Here, we characterized the effect of lamin B mutation on the chromatin structure in D. melanogaster germ cells. We have shown that disruption of the lamin B localization in the nucleus leads to the activation of expression of some retrotransposons and telomeric repeats in the germline, as well as to the increased level of recombination in telomeres. Similar phenomena were observed in Drosophila germ cells during physiological aging, which suggests a close link between the processes occurring in the heterochromatin at the nuclear periphery and the mechanisms of aging.
MATERIALS AND METHODS
D. melanogaster lines. D. melanogaster strain LamA25pr1/CyO with the lamin B mutation was from the Bloomington Drosophila Stock Center (BDSC, USA; no. 25092). The mutation was caused by a frameshift at the C-terminus leading to the deletion of the CaaX motif. The iso-1 (y1; cn1 bw1 sp1) line is an isogenic line used for Drosophila genome sequencing (BDSC, no. 2057).
Western blotting and immunostaining. Total ovarian extracts were fractionated in 8% polyacrylamide gel and transferred to Immobilon-P membrane (Merck Millipore, USA). The signal was visualized with an Immun-Star AP detection system (Bio-Rad, USA). Tissue immunostaining was performed as described previously [24]. The following primary antibodies were used: anti-γ-tubulin (Sigma-Aldrich, USA), anti-lamin C (LC28.26; Developmental Studies Hybridoma Bank, DSHB, USA), anti-lamin B (ADL84.12; DSHB, USA), anti-HOAP [25], anti-γH2Av (Rockland, USA), and anti-Rad51 (GeneTex, USA). Alexa Fluor-conjugated secondary antibodies with the minimal cross-reactivity to IgG of other species (dilution 1 : 500; Jackson ImmunoResearch, USA) were used. DNA was stained with DAPI (4′,6-diamidino-2-phenylindole). DNA fragmentation in Drosophila ovaries was evaluated using the TUNEL assay (terminal deoxynucleotidyl transferase dUTP nick end labeling) according to the manufacturer’s protocol (Click-iT™ TUNEL Alexa Fluor Imaging, Invitrogen, USA).
Immunofluorescent images were obtained with a Zeiss LSM 900 confocal microscope (Germany); the Z-stacks were recorded with a 0.5-µM step. The images were processed with the ImageJ software. To assess the colocalization of γH2Av and Rad51 with the telomeric HOAP protein, 30-40 nuclei from two independent biological samples were analyzed; computer-assisted deconvolution was performed for the optical sections at different levels along the z-axis. Colocalization of signals was evaluated with the ImageJ software using the Colocalization plugin. The overlap threshold has been set to 3 pixels. GraphPad Prism 5.0 (GraphPad Software, San Diego, California USA, https://www.graphpad.com) was used for the statistical analysis and graphing; the pairwise comparison was carried out according to the Mann–Whitney U-test.
Reverse transcription and quantitative PCR. Total RNA was extracted from Drosophila ovaries using ExtractRNA reagent (Evrogen, Russia). Reverse transcription (RT) was carried out with a 6-nucleotide random primer and M-MuLV reverse transcriptase (Biolabmix, Russia). Quantitative PCR (qPCR) was performed in a LightCycler 96 (Roche, Switzerland); digital PCR (dPCR) was performed with a QIAcuity Digital PCR System instrument (Qiagen, USA). Gene-specific primers used for qPCR were: rp49 5′-ATGACCATCCGCCCAGCATAC-3′, 5′-GCTTAGCATATCGATCCGACTGG-3′; Pgd 5′-CCAGAAGGGCACGGGCAA-3′, 5′-CAGGGCAGACAGGCATCGC-3′; HeT-A ORF 5′-GGAGTGATGAGCGGCGGAAA-3′, 5′-CCAGGCAAGCGGACAAACGA-3′; HeT-A promoter 5′-ACCACGCCCAACCCCCAA-3′, 5′ GCTGGTGGAGGTACGGAGACAG-3′; TART-B 5′-CACACCCACACAATATAACGACA-3′, 5′-CTGATTCGCTTGTGAATTGC-3′; GATE 5′-CATCACACGTTGTTGCACCGA-3′, 5′- GCACTGCCAAGAAGGATAGCTCT-3′; light 5′-GAAAGATCAAAATGGGACAGA-3′, 5′-TGAGCATAGTTGTTCGTAGGA-3′; copia 5′-CGACAGTGTGGAGGTTGTGCC-3′, 5′-CTTGGAGACGCTTTACGGACAT-3′; 1731 5′-ATGTTTGTGGAAGGTGGTTTCAGG-3′, 5′-GCTTTTTCATCTTGGGATTGCC-3′; 60D 5′- CCAGCCGAGACGAGCACCATAAT-3′, 5′-TTCCCCATCCTCGAGCCCTG-3′; 42AB 5′-CGTCCCAGCCTACCTAGTCA-3′, 5′-ACTTCCCGGTGAAGACTCCT-3′; 38C1 5′-GATACTGGTTCTACGGTGCGAAAATAC-3′, 5′-GTGCTTGTGTGCTGTGTGAG-3′. The standard error of mean (SEM) was calculated for three biological replicas; the statistical significance was assessed using the Student’s t-test (* p < 0.05 to 0.01, ** p < 0.01 to 0.001, *** p < 0.001).
Chromatin immunoprecipitation (ChIP) was performed according to the previously published protocol [26]. Chromatin isolated from Drosophila ovaries was precipitated with the antibodies against H3 histone trimethylated at Lys9 (H3K9me3; Merck 07-523, Germany). The primers used in ChIP-qPCR are listed in the previous section. qPCR was performed using a LightCycler 96 (Roche). Enrichment in ChIP was calculated as % of the input. The standard error of mean (SEM) was calculated for three biological replicas; the statistical significance was assessed using the Student’s t-test.
RESULTS
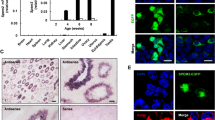
LamA25 mutants are characterized by the germ cell death and defects of oogenesis. The genome of Drosophila contains two lamin genes: LamC encoding lamin of A/C type and Lam (or Dm0) encoding lamin B. Lamins contain the C-terminal CaaX motif required for the protein localization at the inner surface of the nuclear membrane [27]. The LamA25 mutation is caused by a translational frameshift at the C-terminus, which does not significantly change protein molecular weight, but disrupts the CaaX box, leading to the delocalization of the mutant lamin B in somatic cells [28]. Both DNA damage and neuronal cell death were observed in the neuronal tissues of LamA25 mutants [29]. Viable lamin B mutants, including LamA25, develop to the imago stage, but are sterile [23, 28]. We have shown that the ovaries of LamA25 flies were significantly reduced, and oogenesis terminated at the intermediate stages (Fig. 1a). Ovarian immunostaining with antibodies against the N-terminus of lamin B produced a very weak signal corresponding to the mutant protein in the nucleoplasm, while lamin C remained on the nuclear membrane (Fig. 1b). Immunostaining of the telomere-specific HP1/ORC-associated protein (HOAP) showed that the predominant location of telomeres on the periphery of the nucleus was not altered in the LamA25 mutants compared to the control (Fig. 1b). It should be noted that the studied ovarian nurse cells have a polyploid genome, but a low level of chromosomal polyteny. As a result, the number of signals from the telomeres was significantly higher than the number of chromosomal arms. At the same time, a large cluster of telomeres was clearly distinguished on the nuclear periphery, which most likely comprised the heterochromatic arms of the chromosomes. Western blot analysis showed a decrease in the amount of lamin B in the ovaries of LamA25 mutants, which indicated not only lamin B delocalization, but also its destabilization, and confirmed the results of protein immunostaining (Fig. 1, b and c). The TUNEL staining of ovaries, which allows detecting DNA fragmentation revealed the presence of TUNEL-positive cells in the LamA25 mutants. The degeneration of the entire egg chambers (morphological units of oogenesis) was observed (Fig. 1d). Therefore, delocalization and destabilization of lamin B caused accelerated death of germ cells and defects in oogenesis.
The LamA25 mutation of lamin B impairs oogenesis in Drosophila. a) General view of the ovaries of the yw (control) and LamA25 Drosophila flies. b) No lamin B was detected on the nuclear envelope of LamA25 nurse cells. The ovaries were stained with antibodies against telomeric HOAP (red) and lamins C or B (green); DNA was stained with DAPI (blue). c) Western blot analysis of yw and LamA25 ovarian protein extracts using antibodies against lamin B, lamin C, and γ-tubulin (loading control). d) Fragmentation of DNA revealed by TUNEL staining (green) indicates germ cell death and egg chamber degeneration in the ovaries of LamA25 mutants in comparison to the yw control line. Egg chambers at the 4th stage of oogenesis are shown.
Compaction of genomic repeats relaxes and expression of retrotransposons and telomeric repeats increases in the ovaries of LamA25 mutants. To find out whether the chromatin structure was altered in the ovaries of LamA25 mutants, we performed ChIP-qPCR using antibodies against H3K9me3 (histone 3 modification associated with inactive chromatin) and found a significant decrease in the heterochromatin H3K9me3 mark in some retrotransposons and in the heterochromatin loci light, 42AB, and 38C1, which indicated the decompaction of chromatin (Fig. 2a).
Lamin B mutation affects heterochromatin loci, retrotransposons, and telomeres. a) ChIP of the ovarian chromatin of control (yw) and mutant (LamA25) D. melanogaster lines with anti-H3K9me3 antibodies revealed changes in the chromatin state of the telomeric repeats, retrotransposons, and heterochromatin loci. Euchromatic locus 60D was used as a negative control. ChIP enrichment was calculated as % of input. b) Relative levels of transcripts of the HeT-A and TART telomeric repeats and retrotransposons were determined by RT-qPCR with total RNA isolated from the ovaries of heterozygous (LamA25/+) and homozygous (LamA25/LamA25) mutants (normalized to the transcription of the rp49 housekeeping gene). The standard error of mean (SEM) was calculated for three independent experiments. Asterisks indicate statistical significance (* p < 0.05-0.01, ** p < 0.01-0.001, *** p < 0.001) c) Changes (folds) in the content of transcripts of the HeT-A and TART telomeric repeats were determined by RT-dPCR in the ovaries of homozygous LamA25/LamA25 mutants relative to heterozygous LamA25/+ mutants (normalized to transcription of the Pgd housekeeping gene). Standard deviation was calculated for 3 technical replicas. d) Immunostaining of ovaries with the antibodies against HOAP (red) and DNA damage marker γH2Av (green). γH2Av foci are observed in the heterochromatin and telomeres in the nuclei of ovarian nurse cells of LamA25 mutants. Scale bars, 10 µM. e) Immunostaining of ovaries with the antibodies against HOAP (red) and Rad51 recombinase (green). Activation of recombination in the heterochromatin block and telomeres is observed in the nuclei of ovarian nurse cells of LamA25 mutants; DNA was stained with DAPI (blue). Scale bars, 10 µM. f) Statistical analysis of the γH2Av and Rad51 colocalization with HOAP; *** p < 0,0001.
Drosophila telomeres are maintained by retrotranspositions of specialized telomeric retrotransposons to the chromosome ends; the two major retrotransposons are HeT-A and TART [30]. Despite the absence of telomerase, many components and molecular mechanisms for the telomere maintenance in Drosophila are similar to those in the species that use telomerase [31]. It should be noted that one of the most important characteristics of telomeres is their heterochromatic structure and localization on the nuclear periphery [32]. LamA25 mutants showed a decrease in the H3K9me3 modification at the HeT-A and TART telomeric repeats (Fig. 2a). Therefore, delocalization of lamin B leads to the disruption of the structure of heterochromatin, including telomeric heterochromatin, in Drosophila ovaries.
Next, we checked whether the transcriptional activity changed in the regions with the altered chromatin state. Comparison of homo- and heterozygous flies carrying the LamA25 mutation allowed us to minimize the effect of genetic background and associated differences in the copy number of transposable elements and telomeric repeats. Using RT-qPCR, we demonstrated a modest but statistically significant increase in the RNA levels for some retrotransposons and telomeric repeats in the ovaries of LamA25 mutants (Fig. 2b). The derepression of telomeric repeats was confirmed by a more sensitive RT-dPCR assay which also used a different control to normalize the samples. Indeed, the RNA levels for the HeT-A and TART telomeric repeats increased in the ovaries of LamA25 mutants (Fig. 2c).
Markers of DNA damage and homologous recombination are observed at the nuclear periphery in ovarian germ cells of LamA25 mutants. Normally, in ovarian polyploid nurse cells of Drosophila, telomeres are located predominantly at the nuclear periphery [32]. A particularly large cluster of telomeres was observed near the nuclear lamina of the nurse cells within a large block of heterochromatin, which was characterized by a brighter DAPI staining (Figs. 1 and 2). Using immunostaining with antibodies against phosphorylated histone H2Av (γH2Av), which is a marker of double-strand DNA breaks and replicative stress, we assessed how the LamA25 mutation affected the stability of telomeres and of the entire genome in the nuclei of ovarian nurse cells. We observed the accumulation of γH2Av foci, which were especially well seen at the nuclear periphery, in the ovaries of LamA25 mutants. Some signals of HOAP, a protein of the telomere protective complex, overlapped with the signals of γH2Av, which indicated telomere damage or dysfunction of the telomere protection complex (Fig. 2d).
Rad51, a marker of DNA recombination, interacts with a single-stranded DNA at the stage of strand invasion during homologous recombination. In Drosophila cells, the activity of Rad51 is blocked in the repeat-enriched heterochromatin domains in order to prevent ectopic recombination [3]. No Rad51 immunostaining was observed in the control ovaries, while in the LamA25 mutants, Rad51 accumulated at the nuclear periphery of nurse cells. A particularly strong staining was observed within the heterochromatic block, which was distinguished by a bright DAPI staining (Fig. 2e). In this region, the signals of HOAP strongly overlapped with the signal of Rad51. Statistical analysis revealed that colocalization of γH2Av or Rad51 with the telomeric HOAP was more pronounced in the LamA25 mutants compared to the control ovaries (Fig. 2f). Taken together, our data show that the LamA25 mutation disrupts homeostasis at the nuclear periphery, leading to the heterochromatin decompaction followed by the activation of transcription and recombination, which threatens the genome integrity and leads to the germ cell death.
Aging is accompanied by the upregulation of expression of transposable elements and telomeric repeats and appearance of signs of homologous recombination in the heterochromatin of ovarian germ cells in Drosophila. The loss of repressive chromatin marks leading to the transcriptional activation of retrotransposons during aging in yeast and flies has been reported [33, 34]. Hence, we investigated how the chromatin state and transcription levels of telomeric repeats and retrotransposons changed in the Drosophila germline as a result of aging. The average lifespan of natural populations of D. melanogaster is 55 days [35]. In our study, we used the isogenic strain iso-1, whose genome was sequenced and annotated. Analysis of chromatin by ChIP-qPCR using anti-H3K9me3 antibodies revealed a significant decrease in this heterochromatin mark at the telomeric repeats in the ovaries of 50-day-old flies (Fig. 3a). The levels of transcripts for the HeT-A and TART telomeric repeats and some retrotransposons significantly increased in the ovaries of 50-day-old flies compared to 3-day-old flies (Fig. 3b). These results show that aging leads to the chromatin decompaction and transcriptional activation of retrotransposons and telomeric repeats in D. melanogaster ovaries. It should be noted that the extent of changes was comparable to those observed in the LamA25 mutants.
Aging is accompanied by the expression of retrotransposons and telomeric repeats, DNA damage, and appearance of signs of homologous recombination in the heterochromatin of Drosophila ovarian germ cells. a) ChIP in the ovaries of 3-day-old and 50-day-old iso-1 D. melanogaster females using anti-H3K9me3 antibodies revealed changes in the chromatin state in the telomeric repeats, retrotransposons, and heterochromatin loci. b) Relative content of the transcripts for retrotransposons and telomeric repeats was detected by RT-qPCR using total RNA isolated from the ovaries of 3-day-old and 50-day-old iso-1 Drosophila flies. c and d) Immunostaining of the ovaries of 3-day-old and 50-day-old Drosophila flies with the antibodies against HOAP (red), γH2Av (green) (c), and Rad51 (green) (d). The signals of homologous recombination in the telomeres and DNA damage at the nuclear periphery and in the telomeres observed in the nuclei of nurse cells of 50-day-old females. DNA was stained with DAPI (blue). e) Statistical analysis of γH2Av and Rad51 colocalization with HOAP. * p < 0.05-0.01, ** p < 0.01-0.001, *** p < 0,0001 (a, b, e). f) TUNEL staining (green) of the ovaries revealed germ cell death in 50-day-old females compared to 3-day-old females. Egg chambers at stage 6 of oogenesis are shown. Scale bars, 10 µM.
Immunostaining of the ovaries of 3-day-old females showed almost no γH2Av and Rad51 signals, while in the 50-day-old females, numerous signals of γH2Av, a marker of DNA damage, appeared in the nuclei of nurse cells, mainly at the nuclear periphery. Some γH2Av signals overlapped with the signals of HOAP (Fig. 3, c and e). Strong signals of Rad51 recombinase were observed in the heterochromatin regions and telomeres in 50-day-old females (Fig. 3, d and e). TUNEL staining of the ovaries revealed the death of germ cells in 50-days-old females (Fig. 3f).
Therefore, physiological aging is accompanied by the changes in the heterochromatin state at the periphery of germ cell nuclei, that are strikingly similar to those observed in the LamA25 mutants with impaired lamin B localization.
DISCUSSION
Laminopathies are commonly associated with mutations in lamin A leading to the changes in the chromatin organization, abnormal replication and DNA repair, and telomere dysfunction [5, 13, 18]. In many organisms, lamin B performs functions similar to those of lamin A and plays a specific role in the maintenance of neurons [8, 36]. The presence of several types of lamins with similar properties most likely compensates for the dysfunction of one of them, but at the same time, each lamin demonstrates its cellular and functional specialization. Numerous data point to the critical role of lamin B in aging. As organisms age, the content of lamin B gradually decreases due to its destabilization [37, 38], which is the cause of neurodegeneration and immune system disorders in Drosophila [38, 39]. It has also been found that the amount of lamin B1 decreases significantly in senescent mammalian cells and in the process of cell aging [40-42], thus making lamin B level an important marker of aging. The fact that mutations in the prolyl isomerase PIN1 (Dodo protein in Drosophila), which is required to maintain the structure of lamin B, destabilize heterochromatin demonstrates the importance of the structural integrity of lamin B for the heterochromatin maintenance [43]. Here, we used Drosophila flies with the mutation that prevents lamin B localization at the nuclear envelope and results in the decrease in the lamin B content, thus indicating its destabilization, i.e., the processes also observed in aging [38]. LamA25 mutants with the altered localization and decreased content of lamin B are a promising model for studying the mechanisms of aging at the organismal level. It should be noted that LamA25 mutants, like other viable lamin B mutants, develop to the imago stage, but are sterile [23, 28]. This suggests that the control of genome integrity during gametogenesis is very strict in order to ensure the stability of genetic information over generations.
We used LamA25 mutants to study the changes in the nuclei of the germline nurse cells. Despite the presence of lamin C at the nuclear membrane, we observed decompaction of heterochromatin loci, retrotransposons, and telomeres in the LamA25 mutants, which was accompanied by a moderate increase in the levels of transcripts of some transposable elements and telomeric repeats. Immunostaining of the telomere-specific markers in the LamA25 mutants revealed no noticeable shift of telomeres from the nuclear periphery. Apparently, the effect of lamin B mutation was manifested in the disruption of the functional compartment near the nuclear lamina, while lamin C present in the germ cells performed the topological functions. Beside the heterochromatin decompaction and activation of transposons, we observed a strong accumulation of Rad51 recombinase in the heterochromatin after lamin B delocalization, which indicated activation of recombination processes. Rad51 is involved in homologous recombination and is indispensable for repairing DNA damage in the euchromatin; however, its activity in the repeat-rich regions can lead to the ectopic recombination and chromosomal rearrangements [44]. The processes of DNA damage repair and homologous recombination in the heterochromatin are uncoupled by yet poorly understood mechanisms [45, 46]. It has been shown that the safe DNA repair can occur at the nuclear periphery in the regions enriched with HP1 (heterochromatin protein 1) due to the displacement of Rad51 from these regions [47, 48]. This mechanism is aimed at preventing ectopic recombination between the repeats in the heterochromatin. The fact that we observed heterochromatin decompaction, signs of homologous recombination, and DNA damage in the telomeres at the nuclear periphery indicated the destruction of the nuclear infrastructure in the lamin B mutants (Fig. 4). Interestingly, the same signs of homologous recombination in the telomeres and heterochromatin were observed during physiological aging in the Drosophila germline. The accumulation of γH2Av histone in the brain of flies with the mutant PIN1 protein (lamin B stability factor) was explained by the appearance of DNA breaks caused by the transposon mobilization [43]. Lamin mutants also showed the signs of DNA damage, the cause of which remained unknown [29]. Our data indicate that destabilization of lamin B in the germline activates a mechanism likely associated with the ectopic recombination between the genomic repeats.
What is the link between lamin B and recombination? Lamins play an important role in the regulation of DNA repair [5, 49]. It has been shown that in human cells, Rad51 recombinase interacts with lamin B1, thus promoting the repair of the double-strand DNA breaks and survival of irradiated cells [50]. It is possible that in a certain genomic context, lamin B can interfere with the interaction between Rad51 and heterochromatin in order to prevent the mutagenic recombination of repeats during the DNA repair or replication. It is believed that the activation of transcription of retrotransposons may be the cause of cell senescence; however, the direct link between these processes has not yet been proven. Our data suggest that ectopic recombination in the heterochromatin in the lamin B mutants with the impaired protein localization can provoke cell death, which we observed using the TUNEL assay. It is possible that similar processes occur during aging and physiological destabilization of lamin B, leading to the apoptosis or mitotic arrest involving the mechanisms of the DNA damage response. Interestingly, laminopathy, which causes global changes in the heterochromatin, is often compared to telomeropathy, which affects only the telomeres. It can be assumed that telomeres are particularly sensitive regions that respond to the functional alterations of lamins. An increased level of recombination accompanied by the DNA damage (which can lead to the chromosomal fusion) was observed in the telomeres both in the LamA25 mutants and during aging. The breaks in the telomeric DNA, replicative stress, and ectopic recombination of telomeric repeats may trigger cell death in both laminopathy and aging. This phenomenon, which can be defined as the “telomeric checkpoint”, has been observed previously in our study of genetic disorders that cause, among other cellular effects, telomere dysfunction in Drosophila [25]. In the latter case, a mitotic catastrophe and developmental arrest were observed due to the interaction of telomeric ribonucleoprotein complexes with the cell cycle proteins. Further studies are required to elucidate the signaling role of telomeres in the mechanism of cell death in laminopathy and aging.
References
Wood, J. G., and Helfand, S. L. (2013) Chromatin structure and transposable elements in organismal aging, Front. Genet., 4, 274, https://doi.org/10.3389/fgene.2013.00274.
Burla, R., La Torre, M., and Saggio, I. (2016) Mammalian telomeres and their partnership with lamins, Nucleus, 7, 187-202, https://doi.org/10.1080/19491034.2016.1179409.
Amaral, N., Ryu, T., Li, X., and Chiolo, I. (2017) Nuclear dynamics of heterochromatin repair, Trends Genet., 33, 86-100, https://doi.org/10.1016/j.tig.2016.12.004.
Ovsiannikova, N. L., Lavrushkina, S. V., Ivanova, A. V., Mazina, L. M., Zhironkina, O. A., and Kireev, II. (2021) Lamin A as a determinant of mechanical properties of the cell nucleus in health and disease, Biochemistry (Moscow), 86, 1288-1300, https://doi.org/10.1134/S0006297921100102.
Gonzalo, S. (2014) DNA damage and lamins, Adv. Exp. Med. Biol., 773, 377-399, https://doi.org/10.1007/978-1-4899-8032-8_17.
Bellanger, A., Madsen-Osterbye, J., Galigniana, N. M., and Collas, P. (2022) Restructuring of lamina-associated domains in senescence and cancer, Cells, 11, 1846, https://doi.org/10.3390/cells11111846.
Gonzalez-Suarez, I., Redwood, A. B., and Gonzalo, S. (2009) Loss of A-type lamins and genomic instability, Cell Cycle, 8, 3860-3865, https://doi.org/10.4161/cc.8.23.10092.
Camps, J., Erdos, M. R., and Ried, T. (2015) The role of lamin B1 for the maintenance of nuclear structure and function, Nucleus, 6, 8-14, https://doi.org/10.1080/19491034.2014.1003510.
Dechat, T., Pfleghaar, K., Sengupta, K., Shimi, T., Shumaker, D. K., Solimando, L., and Goldman, R. D. (2008) Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin, Genes Dev., 22, 832-853, https://doi.org/10.1101/gad.1652708.
Forsberg, F., Brunet, A., Ali, T. M. L., and Collas, P. (2019) Interplay of lamin A and lamin B LADs on the radial positioning of chromatin, Nucleus, 10, 7-20, https://doi.org/10.1080/19491034.2019.1570810.
Ulianov, S. V., Doronin, S. A., Khrameeva, E. E., Kos, P. I., Luzhin, A. V., Starikov, S. S., Galitsyna, A. A., Nenasheva, V. V., Ilyin, A. A., Flyamer, I. M., Mikhaleva, E. A., Logacheva, M. D., Gelfand, M. S., Chertovich, A. V., Gavrilov, A. A., Razin, S. V., and Shevelyov, Y. Y. (2019) Nuclear lamina integrity is required for proper spatial organization of chromatin in Drosophila, Nat. Commun., 10, 1176, https://doi.org/10.1038/s41467-019-09185-y.
Singh, M., Hunt, C. R., Pandita, R. K., Kumar, R., Yang, C. R., Horikoshi, N., Bachoo, R., Serag, S., Story, M. D., Shay, J. W., Powell, S. N., Gupta, A., Jeffery, J., Pandita, S., Chen, B. P., Deckbar, D., Lobrich, M., Yang, Q., Khanna, K. K., Worman, H. J., et al. (2013) Lamin A/C depletion enhances DNA damage-induced stalled replication fork arrest, Mol. Cell. Biol., 33, 1210-1222, https://doi.org/10.1128/MCB.01676-12.
Graziano, S., Coll-Bonfill, N., Teodoro-Castro, B., Kuppa, S., Jackson, J., Shashkova, E., Mahajan, U., Vindigni, A., Antony, E., and Gonzalo, S. (2021) Lamin A/C recruits ssDNA protective proteins RPA and RAD51 to stalled replication forks to maintain fork stability, J. Biol. Chem., 297, 101301, https://doi.org/10.1016/j.jbc.2021.101301.
Butin-Israeli, V., Adam, S. A., Jain, N., Otte, G. L., Neems, D., Wiesmuller, L., Berger, S. L., and Goldman, R. D. (2015) Role of lamin b1 in chromatin instability, Mol. Cell. Biol., 35, 884-898, https://doi.org/10.1128/MCB.01145-14.
Ikegami, K., Secchia, S., Almakki, O., Lieb, J. D., and Moskowitz, I. P. (2020) Phosphorylated Lamin A/C in the nuclear interior binds active enhancers associated with abnormal Transcription in progeria, Dev. Cell, 52, 699-713 e611, https://doi.org/10.1016/j.devcel.2020.02.011.
Huang, S., Risques, R. A., Martin, G. M., Rabinovitch, P. S., and Oshima, J. (2008) Accelerated telomere shortening and replicative senescence in human fibroblasts overexpressing mutant and wild-type lamin A, Exp. Cell Res., 314, 82-91, https://doi.org/10.1016/j.yexcr.2007.08.004.
Sadaie, M., Salama, R., Carroll, T., Tomimatsu, K., Chandra, T., Young, A. R., Narita, M., Perez-Mancera, P. A., Bennett, D. C., Chong, H., Kimura, H., and Narita, M. (2013) Redistribution of the Lamin B1 genomic binding profile affects rearrangement of heterochromatic domains and SAHF formation during senescence, Genes Dev., 27, 1800-1808, https://doi.org/10.1101/gad.217281.113.
Gonzalez-Suarez, I., Redwood, A. B., Perkins, S. M., Vermolen, B., Lichtensztejin, D., Grotsky, D. A., Morgado-Palacin, L., Gapud, E. J., Sleckman, B. P., Sullivan, T., Sage, J., Stewart, C. L., Mai, S., and Gonzalo, S. (2009) Novel roles for A-type lamins in telomere biology and the DNA damage response pathway, EMBO J., 28, 2414-2427, https://doi.org/10.1038/emboj.2009.196.
Ghosh, S., and Zhou, Z. (2014) Genetics of aging, progeria and lamin disorders, Curr. Opin. Genet. Dev., 26, 41-46, https://doi.org/10.1016/j.gde.2014.05.003.
Chen, H., Zheng, X., Xiao, D., and Zheng, Y. (2016) Age-associated de-repression of retrotransposons in the Drosophila fat body, its potential cause and consequence, Aging Cell, 15, 542-552, https://doi.org/10.1111/acel.12465.
Li, W., Prazak, L., Chatterjee, N., Gruninger, S., Krug, L., Theodorou, D., and Dubnau, J. (2013) Activation of transposable elements during aging and neuronal decline in Drosophila, Nat. Neurosci., 16, 529-531, https://doi.org/10.1038/nn.3368.
Wood, J. G., Jones, B. C., Jiang, N., Chang, C., Hosier, S., Wickremesinghe, P., Garcia, M., Hartnett, D. A., Burhenn, L., Neretti, N., and Helfand, S. L. (2016) Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila, Proc. Natl. Acad. Sci. USA, 113, 11277-11282, https://doi.org/10.1073/pnas.1604621113.
Osouda, S., Nakamura, Y., de Saint Phalle, B., McConnell, M., Horigome, T., Sugiyama, S., Fisher, P. A., and Furukawa, K. (2005) Null mutants of Drosophila B-type lamin Dm(0) show aberrant tissue differentiation rather than obvious nuclear shape distortion or specific defects during cell proliferation, Dev. Biol., 284, 219-232, https://doi.org/10.1016/j.ydbio.2005.05.022.
Morgunova, V., Sukhova, M. M., and Kalmykova, A. (2022) Whole-mount RNA FISH combined with immunofluorescence for the analysis of the telomeric ribonucleoproteins in the Drosophila germline, Methods Mol. Biol., 2509, 157-169, https://doi.org/10.1007/978-1-0716-2380-0_10.
Morgunova, V., Kordyukova, M., Mikhaleva, E. A., Butenko, I., Pobeguts, O. V., and Kalmykova, A. (2021) Loss of telomere silencing is accompanied by dysfunction of Polo kinase and centrosomes during Drosophila oogenesis and early development, PLoS One, 16, e0258156, https://doi.org/10.1371/journal.pone.0258156.
Akulenko, N., Ryazansky, S., Morgunova, V., Komarov, P. A., Olovnikov, I., Vaury, C., Jensen, S., and Kalmykova, A. (2018) Transcriptional and chromatin changes accompanying de novo formation of transgenic piRNA clusters, RNA, 24, 574-584, https://doi.org/10.1261/rna.062851.117.
Rusinol, A. E., and Sinensky, M. S. (2006) Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors, J. Cell Sci., 119, 3265-3272, https://doi.org/10.1242/jcs.03156.
Bondarenko, S. M., and Sharakhov, I. V. (2020) Reorganization of the nuclear architecture in the Drosophila melanogaster Lamin B mutant lacking the CaaX box, Nucleus, 11, 283-298, https://doi.org/10.1080/19491034.2020.1819704.
Frost, B., Bardai, F. H., and Feany, M. B. (2016) Lamin dysfunction mediates neurodegeneration in tauopathies, Curr. Biol., 26, 129-136, https://doi.org/10.1016/j.cub.2015.11.039.
Pardue, M. L., and DeBaryshe, P. G. (2003) Retrotransposons provide an evolutionarily robust non-telomerase mechanism to maintain telomeres, Annu. Rev. Genet., 37, 485-511, https://doi.org/10.1146/annurev.genet.38.072902.093115.
Kordyukova, M., Olovnikov, I., and Kalmykova, A. (2018) Transposon control mechanisms in telomere biology, Curr. Opin. Genet. Dev., 49, 56-62, https://doi.org/10.1016/j.gde.2018.03.002.
Radion, E., Morgunova, V., Ryazansky, S., Akulenko, N., Lavrov, S., Abramov, Y., Komarov, P. A., Glukhov, S. I., Olovnikov, I., and Kalmykova, A. (2018) Key role of piRNAs in telomeric chromatin maintenance and telomere nuclear positioning in Drosophila germline, Epigenetics Chromatin, 11, 40, https://doi.org/10.1186/s13072-018-0210-4.
Dang, W., Steffen, K. K., Perry, R., Dorsey, J. A., Johnson, F. B., Shilatifard, A., Kaeberlein, M., Kennedy, B. K., and Berger, S. L. (2009) Histone H4 lysine 16 acetylation regulates cellular lifespan, Nature, 459, 802-807, https://doi.org/10.1038/nature08085.
Wood, J. G., Hillenmeyer, S., Lawrence, C., Chang, C., Hosier, S., Lightfoot, W., Mukherjee, E., Jiang, N., Schorl, C., Brodsky, A. S., Neretti, N., and Helfand, S. L. (2010) Chromatin remodeling in the aging genome of Drosophila, Aging Cell, 9, 971-978, https://doi.org/10.1111/j.1474-9726.2010.00624.x.
Ivanov, D. K., Escott-Price, V., Ziehm, M., Magwire, M. M., Mackay, T. F., Partridge, L., and Thornton, J. M. (2015) Longevity GWAS using the drosophila genetic reference panel, J. Gerontol. A Biol. Sci. Med. Sci., 70, 1470-1478, https://doi.org/10.1093/gerona/glv047.
Coffinier, C., Jung, H. J., Nobumori, C., Chang, S., Tu, Y., Barnes, R. H., 2nd, Yoshinaga, Y., de Jong, P. J., Vergnes, L., Reue, K., Fong, L. G., and Young, S. G. (2011) Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons, Mol. Biol. Cell, 22, 4683-4693, https://doi.org/10.1091/mbc.E11-06-0504.
Shah, P. P., Donahue, G., Otte, G. L., Capell, B. C., Nelson, D. M., Cao, K., Aggarwala, V., Cruickshanks, H. A., Rai, T. S., McBryan, T., Gregory, B. D., Adams, P. D., and Berger, S. L. (2013) Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape, Genes Dev., 27, 1787-1799, https://doi.org/10.1101/gad.223834.113.
Lin, W. Q., Ngian, Z. K., Koh, T. W., and Ong, C. T. (2022) Altered stability of nuclear lamin-B marks the onset of aging in male Drosophila, PLoS One, 17, e0265223, https://doi.org/10.1371/journal.pone.0265223.
Chen, H., Zheng, X., and Zheng, Y. (2014) Age-associated loss of lamin-B leads to systemic inflammation and gut hyperplasia, Cell, 159, 829-843, https://doi.org/10.1016/j.cell.2014.10.028.
Freund, A., Laberge, R. M., Demaria, M., and Campisi, J. (2012) Lamin B1 loss is a senescence-associated biomarker, Mol. Biol. Cell, 23, 2066-2075, https://doi.org/10.1091/mbc.E11-10-0884.
Shimi, T., Butin-Israeli, V., Adam, S. A., Hamanaka, R. B., Goldman, A. E., Lucas, C. A., Shumaker, D. K., Kosak, S. T., Chandel, N. S., and Goldman, R. D. (2011) The role of nuclear lamin B1 in cell proliferation and senescence, Genes Dev., 25, 2579-2593, https://doi.org/10.1101/gad.179515.111.
Dreesen, O., Chojnowski, A., Ong, P. F., Zhao, T. Y., Common, J. E., Lunny, D., Lane, E. B., Lee, S. J., Vardy, L. A., Stewart, C. L., and Colman, A. (2013) Lamin B1 fluctuations have differential effects on cellular proliferation and senescence, J. Cell Biol., 200, 605-617, https://doi.org/10.1083/jcb.201206121.
Napoletano, F., Ferrari Bravo, G., Voto, I. A. P., Santin, A., Celora, L., Campaner, E., Dezi, C., Bertossi, A., Valentino, E., Santorsola, M., Rustighi, A., Fajner, V., Maspero, E., Ansaloni, F., Cancila, V., Valenti, C. F., Santo, M., Artimagnella, O. B., Finaurini, S., Gioia, U., et al. (2021) The prolyl-isomerase PIN1 is essential for nuclear Lamin-B structure and function and protects heterochromatin under mechanical stress, Cell Rep., 36, 109694, https://doi.org/10.1016/j.celrep.2021.109694.
Kramara, J., Osia, B., and Malkova, A. (2018) Break-induced replication: the where, the why, and the how, Trends Genet., 34, 518-531, https://doi.org/10.1016/j.tig.2018.04.002.
Tsouroula, K., Furst, A., Rogier, M., Heyer, V., Maglott-Roth, A., Ferrand, A., Reina-San-Martin, B., and Soutoglou, E. (2016) Temporal and spatial uncoupling of DNA double strand break repair pathways within mammalian heterochromatin, Mol. Cell, 63, 293-305, https://doi.org/10.1016/j.molcel.2016.06.002.
Merigliano, C., and Chiolo, I. (2021) Multi-scale dynamics of heterochromatin repair, Curr. Opin. Genet. Dev., 71, 206-215, https://doi.org/10.1016/j.gde.2021.09.007.
Chiolo, I., Minoda, A., Colmenares, S. U., Polyzos, A., Costes, S. V., and Karpen, G. H. (2011) Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair, Cell, 144, 732-744, https://doi.org/10.1016/j.cell.2011.02.012.
Ryu, T., Spatola, B., Delabaere, L., Bowlin, K., Hopp, H., Kunitake, R., Karpen, G. H., and Chiolo, I. (2015) Heterochromatic breaks move to the nuclear periphery to continue recombinational repair, Nat. Cell Biol., 17, 1401-1411, https://doi.org/10.1038/ncb3258.
Lambert, M. W. (2019) The functional importance of lamins, actin, myosin, spectrin and the LINC complex in DNA repair, Exp. Biol. Med. (Maywood), 244, 1382-1406, https://doi.org/10.1177/1535370219876651.
Liu, N. A., Sun, J., Kono, K., Horikoshi, Y., Ikura, T., Tong, X., Haraguchi, T., and Tashiro, S. (2015) Regulation of homologous recombinational repair by lamin B1 in radiation-induced DNA damage, FASEB J., 29, 2514-2525, https://doi.org/10.1096/fj.14-265546.
Acknowledgments
The equipment used in the study was from the Collective Use Center of the Institute of Molecular Genetics of National Research Centre “Kurchatov Institute” and Kurchatov Center for Genome Research.
Funding
This work was supported by the Russian Science Foundation (grant no. 22-14-00006 to A.I.K.).
Author information
Authors and Affiliations
Contributions
A. I. Kalmykova developed the concept, supervised the study, and wrote the manuscript; V. V. Morgunova conducted the experiments and performed statistical analysis, L. G. Malaev conducted the experiments, created the figures, and edited the manuscript; O. A. Sokolova conducted the experiments and edited the manuscript; T. V. Sizova, D. S. Babaev, and D. A. Kwon conducted the experiments.
Corresponding author
Ethics declarations
The authors declare no conflict of interest. This article does not contain description of studies with the involvement of humans or animal subjects performed by any of the authors.
Rights and permissions
Open access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morgunova, V.V., Sokolova, O.A., Sizova, T.V. et al. Dysfunction of Lamin B and Physiological Aging Cause Telomere Instability in Drosophila Germline. Biochemistry Moscow 87, 1600–1610 (2022). https://doi.org/10.1134/S000629792212015X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629792212015X