Abstract
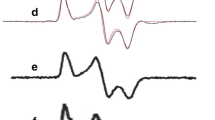
In photosynthetic reaction centers of intact photosystem I (PSI) complexes from cyanobacteria, electron transfer at room temperature occurs along two symmetrical branches of redox cofactors A and B at a ratio of ~3 : 1 in favor of branch A. Previously, this has been indirectly demonstrated using pulsed absorption spectroscopy and more directly by measuring the decay modulation frequencies of electron spin echo signals (electron spin echo envelope modulation, ESEEM), which allows to determine the distance between the separated charges of the primary electron donor P700+ and phylloquinone acceptors A1A– and A1B– in the symmetric redox cofactors branches A and B. In the present work, these distances were determined using ESEEM in PSI complexes lacking three 4Fe–4S clusters, FX, FA, and FB, and the PsaC protein subunit (the so-called P700–A1 core), in which phylloquinone molecules A1A and A1B serve as the terminal electron acceptors. It was shown that in the P700–A1 core preparations, the average distance between the centers of the P700+A1– ion-radical pair at a temperature of 150 K in an aqueous glycerol solution and in a dried trehalose matrix, as well as in a trehalose matrix at 280 K, is ~25.5 Å, which corresponds to the symmetrical electron transfer along the A and B branches of redox cofactors at a ratio of 1 : 1. Possible reasons for the change in the electron transfer symmetry in PSI upon removal of the PsaC subunit and 4Fe–4S clusters FX, FA, and FB are discussed.





Similar content being viewed by others
Abbreviations
- A1 :
-
phylloquinone, secondary electron acceptor
- Chl:
-
chlorophyll
- Em :
-
midpoint redox potential
- ESEEM:
-
electron spin echo envelope modulation
- P700 :
-
chlorophyll dimer, primary electron donor
- PhQ:
-
phylloquinone
- PSI:
-
photosystem I
- RC:
-
reaction center
References
Jordan, P., Fromme, P., Witt, H. T., Klukas, O., Saenger, W., and Krauß, N. (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution, Nature, 411, 909-917, https://doi.org/10.1038/35082000.
Brettel, K., and Leibl, W. (2001) Electron transfer in photosystem I, Biochim. Biophys. Acta, 1507, 100-114, https://doi.org/10.1016/s0005-2728(01)00202-x.
Srinivasan, N., and Golbeck, J. H. (2009) Protein–cofactor interactions in bioenergetic complexes: The role of the A1A and A1B phylloquinones in Photosystem I, Biochim. Biophys. Acta Bioenerg., 1787, 1057-1088, https://doi.org/10.1016/j.bbabio.2009.04.010.
Parrett, K. G., Mehari, T., Warren, P. G., and Golbeck, J. H. (1989) Purification and properties of the intact P-700 and Fx-containing Photosystem I core protein, Biochim. Biophys. Acta, 973, 324-332, https://doi.org/10.1016/S0005-2728(89)80439-6.
Brettel, K., and Golbeck, J. H. (1995) Spectral and kinetic characterization of electron acceptor A1 in a Photosystem I core devoid of iron-sulfur centers FX, FB and FA, Photosynth. Res., 45, 183-193, https://doi.org/10.1007/BF00015559.
Makita, H., and Hastings, G. (2015) Directionality of electron transfer in cyanobacterial photosystem I at 298 and 77 K, FEBS Lett., 589, 1412-1417, https://doi.org/10.1016/j.febslet.2015.04.048.
Joliot, P., and Joliot, A. (1999) In vivo analysis of the electron transfer within photosystem I: Are the two phylloquinones involved? Biochemistry, 38, 11130-11136, https://doi.org/10.1021/bi990857c.
Schlodder, E., Falkenberg, K., Gergeleit, M., and Brettel, K. (1998) Temperature dependence of forward and reverse electron transfer from A1-, the reduced secondary electron acceptor in photosystem I, Biochemistry, 37, 9466-9476, https://doi.org/10.1021/bi973182r.
Guergova-Kuras, M., Boudreaux, B., Joliot, A., Joliot, P., and Redding, K. (2001) Evidence for two active branches for electron transfer in photosystem I, Proc. Natl. Acad. Sci. USA, 98, 4437-4442, https://doi.org/10.1073/pnas.081078898.
Ptushenko, V. V., Cherepanov, D. A., Krishtalik, L. I., and Semenov, A. Y. (2008) Semi-continuum electrostatic calculations of redox potentials in photosystem I, Photosynth. Res., 97, 55-74, https://doi.org/10.1007/s11120-008-9309-y.
Ishikita, H., and Knapp, E.-W. (2003) Redox potential of quinones in both electron transfer branches of photosystem I, J. Biol. Chem., 278, 52002-52011, https://doi.org/10.1074/jbc.M306434200.
Cherepanov, D. A., Milanovsky, G. E., Gopta, O. A., Balasubramanian, R., Bryant, D. A., et al. (2018) Electron–phonon coupling in cyanobacterial photosystem I, J. Phys. Chem. B, 122, 7943-7955, https://doi.org/10.1021/acs.jpcb.8b03906.
Ishikita, H., Stehlik, D., Golbeck, J. H., and Knapp, E.-W. (2006) Electrostatic influence of PsaC protein binding to the PsaA/PsaB heterodimer in photosystem I, Biophys. J., 90, 1081-1089, https://doi.org/10.1529/biophysj.105.069781.
Plato, M., Krauß, N., Fromme, P., and Lubitz, W. (2003) Molecular orbital study of the primary electron donor P700 of photosystem I based on a recent X-ray single crystal structure analysis, Chem. Phys., 294, 483-499, https://doi.org/10.1016/S0301-0104(03)00378-1.
Lubitz, W. (2006) EPR studies of the primary electron donor P700 in photosystem, in Photosystem I, Dordrecht, Springer Netherlands, pp. 245-269, https://doi.org/10.1007/978-1-4020-4256-0_17.
Mula, S., Savitsky, A., Möbius, K., Lubitz, W., Golbeck, J. H., et al. (2012) Incorporation of a high potential quinone reveals that electron transfer in Photosystem I becomes highly asymmetric at low temperature, Photochem. Photobiol. Sci., 11, 946-956, https://doi.org/10.1039/c2pp05340c.
Savitsky, A., Gopta, O., Mamedov, M., Golbeck, J. H., Tikhonov, A., et al. (2010) Alteration of the axial met ligand to electron acceptor A0 in Photosystem I: effect on the generation of P700+ A1− radical pairs as studied by W-band transient EPR, Appl. Magnetic Resonance, 37, 85-102, https://doi.org/10.1007/s00723-009-0052-0.
Sun, J., Hao, S., Radle, M., Xu, W., Shelaev, I., Nadtochenko, V., et al. (2014) Evidence that histidine forms a coordination bond to the A0A and A0B chlorophylls and a second H-bond to the A1A and A1B phylloquinones in M688HPsaA and M668HPsaB variants of Synechocystis sp. PCC 6803, Biochim. Biophys. Acta Bioenerg., 1837, 1362-1375, https://doi.org/10.1016/j.bbabio.2014.04.004.
Kurashov, V., Gorka, M., Milanovsky, G. E., Johnson, T. W., Cherepanov, D. A., et al. (2018) Critical evaluation of electron transfer kinetics in P700–FA/FB, P700–FX, and P700–A1 Photosystem I core complexes in liquid and in trehalose glass, Biochim. Biophys. Acta Bioenerg., 1859, 1288-1301, https://doi.org/10.1016/j.bbabio.2018.09.367.
Milanovsky, G., Gopta, O., Petrova, A., Mamedov, M., Gorka, M., et al. (2019) Multiple pathways of charge recombination revealed by the temperature dependence of electron transfer kinetics in cyanobacterial photosystem I, Biochim. Biophys. Acta Bioenerg., 1860, 601-610, https://doi.org/10.1016/J.BBABIO.2019.06.008.
Malferrari, M., Savitsky, A., Mamedov, M. D., Milanovsky, G. E., Lubitz, W., et al. (2016) Trehalose matrix effects on charge-recombination kinetics in Photosystem I of oxygenic photosynthesis at different dehydration levels, Biochim. Biophys. Acta Bioenerg., 1857, 1440-1454, https://doi.org/10.1016/j.bbabio.2016.05.001.
Shelaev, I., Gorka, M., Savitsky, A., Kurashov, V., Mamedov, M., et al. (2017) Effect of dehydrated trehalose matrix on the kinetics of forward electron transfer reactions in photosystem I, Zeitschr. Physikal. Chemie, 231, 325-345, https://doi.org/10.1515/zpch-2016-0860.
Sukhanov, A. A., Mamedov, M. D., Möbius, K., Semenov, A. Y., and Salikhov, K. M. (2018) The decrease of the ESEEM frequency of P700+A1- ion-radical pair in Photosystem I Embedded in trehalose glassy matrix at room temperature can be explained by acceleration of spin–lattice relaxation, Appl. Magnetic Resonance, 49, 1011-1025, https://doi.org/10.1007/s00723-018-1017-y.
Sukhanov, A. A., Mamedov, M. D., Möbius, K., Semenov, A. Y., and Salikhov, K. M. (2020) Impact of iron–sulfur clusters on the spin–lattice relaxation rate and ESEEM frequency of the oxidized primary donor P700+ and reduced phylloquinone acceptor A1−· in radical pairs in Photosystem I embedded in trehalose glassy matrix, Appl. Magnetic Resonance, 51, 909-924, https://doi.org/10.1007/s00723-020-01210-4.
Savitsky, A., Malferrari, M., Francia, F., Venturoli, G., and Möbius, K. (2010) Bacterial photosynthetic reaction centers in trehalose glasses: coupling between protein conformational dynamics and electron-transfer kinetics as studied by laser-flash and high-field EPR spectroscopies, J. Phys. Chem. B, 114, 12729-12743, https://doi.org/10.1021/jp105801q.
Möbius, K., Savitsky, A., Malferrari, M., Francia, F., Mamedov, M. D., et al. (2020) Soft dynamic confinement of membrane proteins by dehydrated trehalose matrices: high-field EPR and fast-laser studies, Appl. Magnetic Resonance, 51, 773-850, https://doi.org/10.1007/s00723-020-01240-y.
Shen, G., Zhao, J., Reimer, S. K., Antonkine, M. L., Cai, Q., et al. (2002) Assembly of photosystem I. I. Inactivation of the rubA gene encoding a membrane-associated rubredoxin in the cyanobacterium Synechococcus sp. PCC 7002 causes a loss of photosystem I activity, J. Biol. Chem., 277, 20343-20354, https://doi.org/10.1074/jbc.M201103200.
Vassiliev, I. R., Jung, Y. S., Mamedov, M. D., Semenov, A. Yu., and Golbeck, J. H. (1997) Near-IR absorbance changes and electrogenic reactions in the microsecond-to-second time domain in Photosystem I, Biophys. J., 72, 301-315, https://doi.org/10.1016/S0006-3495(97)78669-7.
Salikhov, K. M. (1976) Electron Spin Echo and Its Applications [in Russian] (Semenov, A. G., and Tsvetkov, Y. D., eds) Nauka, Novosibirsk, Siberian branch.
Salikhov, K. M., Kandrashkin, Y. E., and Salikhov, A. K. (1992) Peculiarities of free induction and primary spin echo signals for spin-correlated radical pairs, Appl. Magnetic Resonance, 3, 199-216, https://doi.org/10.1007/BF03166790.
Salikhov, K. M., Khairuzhdinov, I. T., and Zaripov, R. B. (2014) Three-pulse ELDOR theory revisited, Appl. Magnetic Resonance, 45, 573-619, https://doi.org/10.1007/s00723-014-0541-7.
Poluektov, O. G., Niklas, J., and Utschig, L. M. (2019) Spin-correlated radical pairs as quantum sensors of bidirectional ET mechanisms in Photosystem I, J. Phys. Chem. B, 123, 7536-7544, https://doi.org/10.1021/acs.jpcb.9b06636.
Dashdorj, N., Xu, W., Cohen, R. O., Golbeck, J. H., and Savikhin, S. (2005) Asymmetric electron transfer in cyanobacterial Photosystem I: charge separation and secondary electron transfer dynamics of mutations near the primary electron acceptor A0, Biophys. J., 88, 1238-1249, https://doi.org/10.1529/BIOPHYSJ.104.050963.
Acknowledgments
This work is dedicated to the memory of the outstanding scientist in the field of primary photosynthetic reactions – Vladimir Anatolyevich Shuvalov. V. A. Shuvalov made an invaluable contribution to the study of the mechanisms of primary reactions in PSI, including the mechanisms of the phylloquinone acceptor A1 reduction. Three co-authors of this article (M.M., G.M., and A.S.) were co-authors of many works by V. A. Shuvalov over the past 15 years and gratefully recall the period of their collaboration with V. A. Shuvalov as exceptionally interesting and productive.
Funding
The study was supported by the Russian Science Foundation and by the Cabinet of Ministers of the Republic of Tatarstan within the framework of the scientific project no. 22-23-20165 (https://rscf.ru/project/22-23-20165/).
Author information
Authors and Affiliations
Contributions
M. D. Mamedov and G. E. Milanovsky isolated PSI complexes; A. A. Sukhanov and K. M. Salikhov recorded EPR spectra; A. Yu. Semenov developed the concept, supervised the study, and wrote the article; M. D. Mamedov, G. E. Milanovsky, A. A. Sukhanov, K. M. Salikhov, and A. Yu. Semenov discussed the results and edited the manuscript.
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. This article does not contain description of studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sukhanov, A.A., Mamedov, M.D., Milanovsky, G.E. et al. Changes in the Electron Transfer Symmetry in the Photosystem I Reaction Centers upon Removal of Iron–Sulfur Clusters. Biochemistry Moscow 87, 1109–1118 (2022). https://doi.org/10.1134/S0006297922100042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297922100042