Abstract
Semaphorin 3A is a secreted glycoprotein, which was originally identified as axon guidance factor in the neuronal system, but it also possesses immunoregulatory properties. Here, the effect of semaphorin 3A on T-lymphocytes, myeloid dendritic cells and macrophages is systematically analyzed on the bases of all publications available in the literature for 20 years. Expression of semaphorin 3A receptors – neuropilin-1 and plexins A – in these cells is described in details. The data obtained on human and murine cells is described comparatively. A comprehensive overview of the interaction of semaphorin 3A with mononuclear phagocyte system is presented for the first time. Semaphorin 3A signaling mostly results in changes of the cytoskeletal machinery and cellular morphology that regulate pathways involved in migration, adhesion, and cell–cell cooperation of immune cells. Accumulating evidence indicates that this factor is crucially involved in various phases of immune responses, including initiation phase, antigen presentation, effector T cell function, inflammation phase, macrophage activation, and polarization. In recent years, interest in this field has increased significantly because semaphorin 3A is associated with many human diseases and therefore can be used as a target for their treatment. Its involvement in the immune responses is important to study, because semaphorin 3A and its receptors turn to be a promising new therapeutic tools to be applied in many autoimmune, allergic, and oncology diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In 2001, just over 20 years ago, the first study devoted to immunomodulatory effects of semaphorin 3A was published [1]. Since then, this protein has occupied one of the main places among “immune semaphorins”. Over the last decade, interest in this factor has grown: the PubMed resource retrieves annually more than 60 publications on semaphorin 3A and 120 – on its receptor neuropilin-1 (Nrp-1). An increasing attention to this factor may be explained by the fact that it participates in the pathogenesis of autoimmune, allergic, as well as oncology diseases, and could be successfully used in their treatment in animals [2-4].
Semaphorin 3A is a glycoprotein secreted by multiple human cell types. From immunological point of view, this is a rather unusual factor exerting simultaneously neuronal and immunoregulatory properties, and additionally acting as an anti-angiogenic agent. One of the typical features related to semaphorin 3A is that it could exhibit a chemorepellent activity so that the directed cell migration occurs down the gradient, i.e., away from the stimulus.
In addition, the structure and function of semaphorin 3A also remarkably differ from other immunomodulatory molecules. For example, semaphorin 3A is structurally similar to its receptor plexin implying their common evolutionary origin. Two rather than one receptor assembled into a single functional complex (holoreceptor) are necessary for the interaction of cells with semaphorin 3A, one of the receptors – Nrp-1 being necessary for ligand binding, while the other – plexin A ensures subsequent signal transduction.
Signaling pathways also exert unique features, since plexin is the only one receptor possessing GAP domain. It interacts with G-proteins that regulate adhesion, migration, proliferation, or survival in diverse cell types.
Quite a lot of reviews dedicated to immune semaphorins of various classes are available, with some of them discussing semaphorin 3A rather briefly [5-7], others describing its role mostly in pathology [2, 3], or providing the data solely on its receptor Nrp-1 [8, 9].
Here, we focus only on semaphorin 3A and its physiological role in the immune system. Systematic analysis of all available publications describing the influence of semaphorin 3A on T cells, myeloid dendritic cells (DCs), and macrophages is presented. A detailed information on the expression of semaphorin 3A receptors on such cell types is provided. The data obtained on human and mouse cells is described comparatively. Here, for the first time, we present a comprehensive overview of the semaphorin 3A action on mononuclear phagocytes.
CLASSIFICATION
Semaphorin 3A belongs to the large semaphorin family which comprises 30 glycoproteins subdivided into 8 classes [5]. The first two classes (1-2) contain semaphorins identified in invertebrates, classes 3-7 – in vertebrates (fishes, birds, amphibians, and mammals), and the final group V is encoded by viruses. No semaphorins are found in single-cell eukaryotes and prokaryotes.
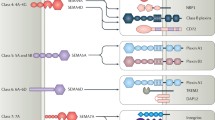
All semaphorins contain highly conserved N-terminal semaphorin (Sema) domain, which is also found in plexins (semaphorin receptors) and in tyrosine kinase receptors MET and RON, receptors of hepatocyte growth factor (HGF) and HGF-like protein (HGFl), respectively. All three protein families (semaphorins, plexins, as well as MET and RON) comprise the semaphorin superfamily (Fig. 1).
Whereas the N-terminal end in all semaphorins is structurally similar, C-terminus diverges, and its structure is essential for binding to cellular membrane. Semaphorins are soluble, intrinsic membrane, or GPI-anchored proteins.
Class 3 semaphorins differ from other vertebrate semaphorins in that they are secreted and have a positively charged basic C-terminal domain. This semaphorin class includes 7 protein isoforms (3A-3H), among them semaphorin 3A is the most studied.
IDENTIFICATION AND STRUCTURE
Class 3 semaphorins were originally identified as axon guidance molecules. In 1990, Raper and colleagues isolated a protein from embryonic chick brain that induced the collapse of neuronal growth cone in culture and was called collapsin-1 [10], but later renamed semaphorin 3A.
Semaphorin 3A is a homodimer consisting of 110-kDa monomeric subunits with a 4 domain structure.
Like all other semaphorins, semaphorin 3A possesses a conserved N-terminal Sema domain. The central feature of this structure is a disulfide-rich seven-blade β-propeller fold. The β-propeller is a very widely used protein fold topology, occurring in both extracellular and cytosolic proteins, but the Sema domain is the largest known variant of this kind, containing approximately 500 amino acids.
Next to the Sema-domain, a small cysteine-rich plexin-semaphorin-integrin (PSI) domain is located following by an immunoglobulin (Ig)-like domain. Basic domain is found at the C-terminus of the molecule.
The Sema-domain is essential for semaphorin-mediated signaling and biological specificity, Ig-like domains linked by disulfide bridge mechanically stabilize homodimeric molecule, whereas C-terminus determines affinity to Nrp-1 receptor [3, 5, 6].
RECEPTORS
To interact with the cell, semaphorin 3A needs a functional receptor complex consisting of Nrp-1 and class A plexin.
Nrp-1 (CD304) is a transmembrane protein with molecular weight of 120-130 kDa. The extracellular part of Nrp-1 consists of two complement-binding domains a1/a2 (CUB domain), two coagulation factor V/VIII homology domains – b1/b2, and c-domain (MAM, meprin) (Fig. 2). Nrp-1 contains a short cytoplasmic domain (about 40 amino acids long) bearing no apparent signaling sequences, hence, signal transduction requires complex formation between Nrp-1 and class A plexins (PlexA1-A4).
Within this complex, Nrp-1 acts as a semaphorin 3A binding receptor, whereas plexin transduces signals inside the cells. During signal transduction a holoreceptor is formed, comprising from two molecules of Nrp-1 and two molecules of PlexA assembled into a single functional complex together with a homodimeric semaphorin 3A [11]. In this complex, both PlexA molecules could be presented as homologous (e.g., PlexA2 dimer) or heterologous (e.g., PlexA1 and PlexA4) counterparts, which is determined by their level of expression in the cell [3]. Moreover, they can even replace each other indicating that there is a significant level of plasticity that enables signal transduction under diverse conditions.
Class A plexins are large transmembrane glycoproteins with molecular weight of around 200 kDa composed of extra- and intracellular parts. The extracellular region is structurally similar to that of semaphorin 3A consisting of the N-terminal Sema-domain; it is followed by three PSI domains and three IPT domains (Ig-like, plexin and transcription factor).
During interaction, semaphorin 3A binds Nrp-1 divalently: Sema domain of semaphorin 3A binds to a1 domain of Nrp-1 with low affinity and basic domain of semaphorin 3A binds to b1 domain of Nrp-1 with high affinity (Fig. 2). The latter occurs only after semaphorin 3A has been processed by a tissue endopeptidase furin [12]. The proteolytic processing is needed for class 3 semaphorin maturation, these proteins being produced in an inactive form. Furin-cleavage pattering allows to exposure of the C-terminal arginine residue, necessary for the interaction with b1 domain of Nrp-1.
Since so far, no physiological high affinity binding between the Sema domains of semaphorin 3A and plexins has been revealed, however weak electrostatic interactions between them may occur (head-to-head) and promote receptor dimerization and activation [12].
SEMAPHORIN 3A SIGNALING
Cytoplasmic part of PlexA has no tyrosine kinase domain typical to growth factor receptors, but has a highly conserved GAP-domain (GTPase activating protein). These regulatory molecules are able to bind activated G-proteins and stimulate their GTPase activity, i.e., accelerate GTP dephosphorylation resulting in GDP formation, which subsequently disrupts signaling event.
Cytoplasmic part of PlexA is capable of activating small monomeric GTPases, G-proteins belonging to the Ras and Rho families. These molecules are well known regulators of adhesion, migration, proliferation, differentiation, and survival of cells. Semaphorin 3A signaling has been most studied in detail in the nervous system and involves GTPases: R-Ras, M-Ras, and Rap (from the Ras family) as well as RhoA, Rac1, and Rnd (from the Rho family).
Ras and Rap GTPases influence integrin function and control cell adhesion, whereas Rho proteins affect cell morphology and motility playing a crucial role in actin cytoskeleton rearrangements similar to Ras proteins [5]. R-Ras and Rap1 activation could initiate diverse inhibitory effects of semaphorin 3A in the nervous and cardiovascular tissues [5, 12]. In the immune system, Rap1 activation is involved in the semaphorin 3A-mediated suppression of human T cell proliferation [13] (Table).
In the inactive state, two GAP domains of plexin are separated by RBD (Rho GTPase binding domain), which per se acts as a Rho binding GTPase (Fig. 2). Upon interaction with semaphorin, this binding results in conformational changes, which allow the separated GAP domains to interact with each other and become activated. Hence, both RBD and GAP domains interact with corresponding G-proteins to trigger complex signaling cascades, which may result not only in inhibitory but also in stimulatory effects.
Rho GTPases are also involved in the PlexA1-mediated actin cytoskeleton rearrangements in DCs that are associated with improvement in DC-T cell crosstalk [14]. The same GTPases also participate in PlexA4-dependent activation of pro-inflammatory activity of macrophages in the presence of lipopolysaccharide (LPS) [15] (Table). Moreover, semaphorin 3A enhances migratory activity of DCs [16] and macrophages [17] through RhoA-ROCK (Rho-associated coiled-coil-forming serine/threonine protein kinase) signaling pathways. If RhoA GTPases become activated by another stimulus, such as macrophage colony-stimulating factor (M-CSF) e.g., semaphorin 3A can downregulate this effect and inhibit macrophage migration [18].
Small GTPases involved in semaphorin 3A signaling in immune cells
Receptor from Nrp-1/PlexA complex | Cell type | Signaling molecules | Function | References |
|---|---|---|---|---|
Nrp-1 | human T lymphocytes | Rap1 | inhibition of proliferation and cytokine production | [13] |
PlexA1 | mouse DCs | Rho/ROCK-kinase | maintaining the ability of DCs to stimulate T cells* | [14] |
Nrp-1/PlexA1 | mouse DCs | RhoA/ROCK-kinase | enhancement of transmigration across lymphatic endothelium | [16] |
Nrp-1 | mouse macrophages | RhoA/ROCK-kinase | stimulation of macrophage migration | [17] |
PlexA4 | mouse macrophages | Rac1 | upregulation of LPS-induced production of inflammatory cytokines | [15] |
Nrp-1 | mouse macrophages | downregulation of M-CSF-activated RhoA pathway | suppression of M-CSF-induced migration | [18] |
In all the cases noted above, as well as in many other situations, actin cytoskeleton and focal adhesion are the main targets of semaphorin 3A. These changes influence cell morphology, cell–cell interactions, adhesion and migration of T cells [19-21] as well as of DCs [14, 16, 22].
In addition to small GTPases, alternative signal transduction pathways were described in neuronal but not yet in immune cells. Direct influence of semaphorin 3A on the cytoskeleton could be also accomplished via interaction of PlexA cytoplasmic tail with two groups of molecules – MICAL (molecules interacting with CasL) and non-receptor intracellular tyrosine kinases Fes and Fyn [3]. Members of the MICAL family are enzymes with monooxygenase activity. They oxidize actin causing actin filament severing and decreased polymerization. Moreover, MICAL proteins, as well as Fes and Fyn tyrosine kinases, can phosphorylate the signaling molecule CRMP2 (collapsin response mediator protein 2), which results in the tubulin depolymerization and suppression of microtubule assembly. Therefore, these changes affect cellular morphology and axon guidance [5].
CRMP2 is found in the cytoplasm of CD4+ and CD8+ T cells as well as of human peripheral blood monocytes [23]. CRMP2 plays a substantial role in the bipolar cytoskeleton reorganization during spontaneous and chemokine-induced T cell migration, however, it is not involved in the semaphorin 3A-mediated signal transduction that inhibits migration of these cells [23].
In addition, there are some other mechanisms of the cellular effects of semaphorin 3A, in particular those related to the development of apoptosis.
It has been demonstrated, that semaphorin 3A can enhance apoptosis in T cell lines [24], as well as in human [25] and rat macrophages [26]. This occurs with the participation of caspases [26].
RECEPTOR FUNCTIONS UNRELATED TO SEMAPHORIN 3A
Nrp-1 is a multifunctional transmembrane protein able to interact with variety of structurally unrelated ligands. Nrp-1 can bind via its b1/b2 domains numerous growth factors such as vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), placental growth factor (PLGF), hepatocyte growth factor (HGF), keratinocyte growth factor (KGF), platelet-derived growth factor-D (PDGF-D), etc. In the process, Nrp-1 forms functional complexes (holoreceptors) with tyrosine kinase receptors of growth factors and affects the downstream signaling [3, 8].
Nrp-1 ligands also include heparin and heparan sulfate, galectin-1, and microRNAs [7, 8]. Nrp-1 can also bind complement components C4d, C3d, and iC3b, that is mediated through b1 domain [27]. Recently, it has been shown that Nrp-1 interacts with S-protein of SARS-CoV-2, facilitating cell infection [28]. All of the above allows us to characterize Nrp-1 as a scaffold receptor [3].
Class A plexins are also not specific exclusively to semaphorin 3A. They can interact with other class 3 semaphorins, such as semaphorin 3B and 3C [3] as well as with class 6 semaphorins [5, 29].
Regardless of the presence or absence of semaphorin 3A, some plexins can participate in macrophage activation and differentiation. For instance, PlexA4 is involved in the TLR (Toll-like receptor) signaling via unknown mechanism [15], whereas PlexA1 takes part in the RANKL-dependent osteoclast differentiation [18] (see “Macrophage” section).
Another unique feature of Nrp-1 and class A plexins is that they can function as independent adhesion molecules. Nrp-1 and PlexA were initially described as such molecules [30, 31]. It has been shown on different non-immune cell types that Nrp-1 interacts with integrins (beta1, αVβ3 and α5β1) and other adhesion molecules (e.g., L1-CAM, L1-cell adhesion molecule) located laterally on the membrane of the same cell, and by doing so, affects the processes of adhesion to extracellular matrix and migration [3, 9, 32]. In the immune system, Nrp-1 and PlexA1, also independently of the presence of semaphorin 3A, participate in the thymocyte adhesion to mouse thymic epithelial cells [33].
Involvement of Nrp-1 and PlexA1 in the formation of immune synapse between naïve T cells and antigen-presenting cells elicits a growing interest. Both receptors are found within the contact zone on DCs and T lymphocytes of human [19, 20, 34] and mouse origin [14, 35]. The possibility of homophilic interaction between two Nrp-1 molecules has been described, where one of them is located on T lymphocyte, and another – on DC surface; introduction of blocking anti-Nrp-1 antibodies inhibits conjugate formation [34]. Nrp-1 prolongs contacts between regulatory T cells (Tregs) and antigen-presenting cells [35], maintains stability and functioning of Tregs [36], facilitates signaling of TGF- β – a key factor for Treg induction [37].
Thus, Nrp-1 and PlexA act not only as semaphorin 3A receptors, but are also engaged in the formation of cell-cell contacts in the immune system, independently of the presence or absence of their main ligand.
SEMAPHORIN 3A EXPRESSION
Semaphorin 3A plays an important role during embryogenesis and participates in the developing of such major systems as: central nervous, cardiovascular, bronchopulmonary, excretory, bone, and immune.
In adults, semaphorin 3A is involved in functioning and maintaining homeostasis of many organs, participates in diverse regenerative processes. It is expressed in human nervous, lymphoid, bone, adipose, and connective tissues, vascular endothelium, gut and nasopharyngeal epitheliums [6].
In the human immune system, semaphorin 3A is produced by activated CD4+ and CD8+ T cells [13, 38], Tregs [38, 39], and CD19+CD25high Bregs [2], as well as by macrophages [19, 25, 38] and DCs [19, 20]. In mice, semaphorin 3A mRNA is found in T and B lymphocytes, NK cells, macrophages and DCs [15].
The main targets of semaphorin 3A in the immune system are: T cells, mononuclear phagocytes and DCs, expressing surface receptor complex composed of Nrp-1 and class A plexins [4].
T CELLS
The major immunological effect of semaphorin 3A is a suppression of T lymphocytes. It is suggested that the biological role of semaphorin 3A is a negative control of T cell immunity [19] realized via a selective downregulation of activated T lymphocytes expressing at this moment semaphorin 3A receptors.
Nrp-1 is practically not detected (0.1-0.5%) by flow cytometry on human peripheral blood mononuclear cells (PBMC), CD4+, CD8+, or CD3+ T lymphocytes [20, 40-42]. However, low level of expression has been found on T cells isolated from the secondary lymphoid tissues such as peripheral lymph nodes (2.6%) and tonsils (5.4%) [40]. Much higher percentage of Nrp-1+ cells is observed in pathology what is documented among CD4+ blood T cells [38] and CD3+ T cells from synovial tissue in patients with rheumatoid arthritis [43] as well as among CD4+ and CD8+ T cells isolated from several human tumors [21, 41, 42].
This data allows us to suggest that Nrp-1 could serve as an activation marker for human T cells [40, 41]. In these cells, Nrp-1 is co-expressed with other surface activation molecules, particularly CD25 that has been shown for CD4+ and CD8+ T cells isolated from tumors [41, 42]. Moreover, Nrp-1 expression is upregulated in peripheral blood T cells after in vitro stimulation with anti-CD3/CD28 antibodies [38, 40].
Nrp-1 is also not determined in mouse T-lymphocytes from blood, spleen or lymph nodes [35, 44], but is present on CD4+ and CD8+ T cells isolated from tumors [21, 41] as well as on peptide-activated CD8+ T-lymphocytes [21].
PlexA1 is poorly expressed on the membrane of resting human T cells, the receptor being located mainly in intracellular compartments. However, during interaction with DCs it is efficiently recruited towards the stimulatory interface in about 50% of conjugating T cells [20]. Low levels of surface PlexA4 can be found on resting T cells [20]. However, PlexA1 and PlexA4 mRNA expression is markedly higher in blood CD4+ and CD8+ T cells from patients with rheumatoid arthritis compared to healthy volunteers [38].
Similar pattern is observed in mice – low mRNA expression of PlexA1 [35, 45], PlexA2, and PlexA4 [15, 21, 46] in non-activated T cells, whereas surface PlexA1 expression is substantially upregulated on peptide-activated CD8+ T cells [21].
Thus, semaphorin 3A is unable to interact with resting lymphocytes, but acquires the ability to influence T cells when they are activated.
In humans, Nrp-1 and PlexA1 are involved in formation of the immunological synapse being expressed on T cells. Herewith, both Nrp-1 [34] and PlexA1 co-localize with CD3 [20]. Exogenous semaphorin 3A suppresses CD3 translocation to the contact zone and causes a transient loss of F-actin content and microvillar extensions in T cells, thus lowering the frequency of conjugates formed between the T cells and DCs [20]; albeit another study does not confirm the effect of semaphorin 3A on cluster formation [19]. Semaphorin 3A triggers actin cytoskeleton rearrangement and talin redistribution, abrogates TCR polarization and early signal transduction events including ZAP-70 and FAK (focal adhesion kinase) phosphorylation. These effects result results in downregulation of DC-induced T cell proliferation [19].
The source of semaphorin 3A in this case are activated DCs involved in antigen presentation. Supernatants from human DC–T cell cocultures (mixed leukocyte reaction) contain small level of semaphorin 3A (5 ng/ml) after one day of culture, whereas on day 4-6 its concentration rises up to 60 ng/ml as documented by immunoprecipitation and immunoblotting [19]. Another study revealed that semaphorin 3A was detected in similar settings on day 3 [20]. Immunosuppressive action of endogenously secreted semaphorin 3A in DC/T cell cocultures (inhibition of allogeneic T cell proliferation) can be blocked with neutralizing anti-semaphorin 3A antibodies [19].
In addition to the suppression of initial phase of immune responses, semaphorin 3A negatively acts on other T cell functions – proliferation and cytokine production. In vitro experiments demonstrate that semaphorin 3A inhibits proliferative activity of human T lymphocytes induced by anti-CD3/CD28 antibodies [13, 19], but does not affect proliferation induced by PHA/IL-2 [19] or phorbol ether [13]. Inhibition of T lymphocyte proliferation is accompanied by the induction of p27kip1 molecule synthesis, which is a negative regulator of the cell cycle. Semaphorin 3A also downregulates IL-2, IL-4, IL-10, and IFN-γ cytokine production, as has been shown on human mononuclear cells stimulated by anti-CD3/CD28 antibodies [13, 39]. The inhibitory effect of semaphorin 3A in T cells is mediated by inhibition of ERK1/2 kinase activation and blockade of Ras/MAPK (mitogen activated protein kinase) signaling pathway via activation of small GTPase Rap1 [13].
Semaphorin 3A also negatively regulates mouse T cell-mediated immune responses. Semaphorin 3A-deficient animals (Sema 3A–/–) exhibit hyperproliferative T cell response to antigen-specific or anti-CD3-mediated stimulation in vitro [46]. Furthermore, this hyperproliferation is also observed in both T cells from Nrp-1 mutant (Nrp-1Sema–) mice, in which the binding site of class 3 semaphorins is disrupted, and in T cells from plexinA4-deficient (PlexA4–/–) mice. Stimulated PlexA4–/– T cells secrete larger amounts of inflammatory cytokines IFN-γ and IL-17 compared with wild-type T cells. The authors suggest that in mice, PlexA4 appears to be a major transducer of negative signals from semaphorin 3A in T cells [46].
It has also been reported that semaphorin 3A downregulates migration and adhesion of T-lymphocytes. It has been shown that this factor inhibits spontaneous migration of PHA/IL-2 preactivated T lymphocytes [23], but does not affect chemokine-induced migration of non-activated human blood T cells [20]. Moreover, semaphorin 3A decreases T cell adhesion to tumor cells [13] and cytotoxic activity of lymphocytes from MLC (mixed lymphocyte cultures) against K562 cells [13]. In mice, semaphorin 3A induces cytoskeletal paralysis of CD8+ T lymphocytes, which leads to suppression of their migration, adhesion, and tumor killing [21, 41].
Moreover, this factor triggers a proapoptotic program that sensitizes human leukemic T cells to Fas (CD95)-mediated apoptosis. In this case, PlexA1 is involved in the transport of actin-linking proteins, cytoskeletal remodeling and Fas translocation into lipid raft microdomains [24].
In addition to direct effects on T-lymphocytes, semaphorin 3A can suppress their activity via activation of Tregs. These cells express transcription factor Foxp3 (forkhead box p3) and are able to inhibit effector T lymphocytes via production of anti-inflammatory cytokines such as TGF-β and IL-10. It is suggested that semaphorin 3A could increase their regulatory properties [38, 39]. This factor is not only expressed by them at mRNA level [38] and in a membrane-bound form [2, 39], but it also increases Foxp3 expression [39] and IL-10 production [38], promoting suppressive activity of CD4+Nrp-1+ Treg-like cells.
Considering that Nrp-1 is practically absent on human blood CD3+CD4+Foxp3+CD25+ Tregs, but is detected on cells isolated from the secondary lymphoid tissues such as lymph nodes and tonsils [40, 47], it has been suggested that Nrp-1 is a marker of a special subset of Tregs. These cells have the most pronounced suppressive potential, and are detected in tissues rather than in peripheral blood; their number is increasing in the conditions of inflammatory microenvironment [8, 48]. In particular, they were identified in the synovial fluid of patients with rheumatoid arthritis [49], in bronchoalveolar lavage fluid from patients with chronic obstructive pulmonary disease [50], as well as in the primary tumors and lymphogenic metastases [42, 47, 48].
At the same time, in mice, Nrp-1 expression on Tregs differs significantly from that in humans. Nrp-1 is highly expressed on CD4+CD25+Foxp3+ cells from peripheral blood, spleen, thymus, and lymph nodes [40], and is considered to be an important functional marker of murine T-regs [35]. Its expression closely correlates with the level of Foxp3 and suppressor function of Tregs [51]. Additionally, Nrp-1 expression defines a subset of thymic Tregs (tTregs) rather than Tregs being induced in periphery (iTregs) [52].
Conditional knockout of Nrp-1 in T cells does not change the number of spleen Tregs [44] or their Foxp3 expression [53]. However, these cells acquire a significant functional defect – reduced ability to suppress effector CD4+ T cell proliferation both in vivo and in vitro. At the same time, CD4+ T cells differentiate preferentially via Th17 lineage commitment. The lack of Nrp1 on the membrane of Tregs results in increased development of autoimmune disorders [53]. Some studies suggest that Nrp-1 is not involved in T cell homeostasis under normal conditions, but its role crucially enhances with the development of pathology [21].
Thus, semaphorin 3A is currently considered as an endogenous immunosuppressor for T lymphocytes. Upon that, it acts selectively only on a small subset of receptor-bearing T cells, among them Tregs, which potentiate semaphoin 3A negative effects on effector T cells. This factor mediates its suppressive activity on activated T cells via its receptors – Nrp-1, PlexA1, and PlexA4 – by blockade of ERK1/2 kinase and Ras/MAPK signaling pathways.
Apart from peripheral immune cells, expression of semaphorin 3A is also detected in the primary lymphoid organ – the thymus. This factor is constitutively produced by human [54] and murine [55] thymic stromal cells. In vitro experiments demonstrates that semaphorin 3A exerts several negative effects on thymocytes and epithelial cells: inhibits adhesion of human thymocytes to epithelial cells [54] as well as SDF-1/CXCL12-induced thymocyte migration. In doing so, the neuronal factor downregulates SDF-1 receptor expression and suppresses phosphorylation of FAK and ZAP-70 kinases in migrating cells, which indicates the existence of the functional antagonism between SDF-1 and semaphorin 3A [56]. Moreover, this factor inhibits proliferative potential of murine thymocytes [57] and thymic epithelial cells [58]. In the latter case, semaphorin 3A is able to abrogate the stimulatory effects of growth factors KGF and HGF on epithelial cell proliferation, thereby allowing to consider semaphorin 3A as a potential antagonist for these two factors also.
DENDRITIC CELLS
DCs not only actively synthesize semaphorin 3A, but represent themselves the targets for the action of semaphorin 3A produced by other cell types. Apart from the suppressive effects on T-lymphocytes, semaphorin 3A stimulates functional activity of DCs and plays an important role in initiating immune response. In this process, the neuronal factor acts rather selectively by mainly affecting DC migration and, potentially, their contact with T lymphocytes, without affecting the process of antigen presentation itself.
This review includes studies performed on myeloid DCs derived from mouse bone-marrow or human peripheral blood monocytes, which were incubated in the presence of GM-CSF and IL-4 (immature DCs) followed by activation with LPS, TNF, or anti-CD40 (mature DCs).
Semaphorin 3A mRNA is poorly expressed in human immature DCs, whereas its expression markedly elevates in the mature DCs activated by LPS, TNF-α/IL-1β, or CD40L [19, 22]. Murine DCs also express semaphorin 3A mRNA [15].
Nrp-1 is usually not expressed by human peripheral blood monocytes [19, 22]. These cells also demonstrate very low level of PlexA1 and PlexA3 expression [22]. The expression of all three receptors becomes markedly upregulated during transformation of monocytes into immature DCs [22, 34]. However, further DC maturation in the presence of LPS is characterized by a decrease of membrane Nrp-1 and PlexA1 expression, what happens due to receptor endocytosis. Upon that, the expression of Nrp-1 mRNA is also downregulated, whereas the level of PlexA1 mRNA remains unaltered [20, 22].
Murine DCs similarly express Nrp-1 [16, 44]. They are also positive for PlexA1 mRNA, whereas other class A plexins show only weak expression [29]. During maturation in the presence of LPS or TNF, murine DCs, unlike human cells, upregulate the expression of Nrp-1 [16, 44] and PlexA1 both at the surface and mRNA levels [14, 16, 45]. In the process, the cytoplasmic PlexA1 becomes primarily located on the cell membrane [14].
Significant interspecies differences in semaphorin 3A receptor expression between the human and murine cells are found not only for DCs, but also for thymocytes [57, 59] and peripheral blood T lymphocytes [40].
PlexA1 is the major plexin A receptor highly expressed on DCs. It is restricted mainly to these cells, indicating PlexA1 is a marker that can distinguish DCs from other antigen-presenting cells, in particular, macrophages [45].
Another receptor – PlexA4, plays no essential role in antigen presentation and the ability of murine DCs to activate T lymphocytes [15]. PlexA4-deficiency does not affect expression of the major histocompatibility complex class II (MHC-II) antigens or costimulatory molecules on DCs, and even promotes their capacity to activate proliferation of allogeneic T lymphocytes [46]. However, PlexA4 still participates in regulation of some functions of DCs, such as cytokine production. DCs from PlexA4–/– mice, activated by LPS, exhibit lower capacity to produce TNF-α and IL-6 [15], albeit the level of IL-12p40 under similar conditions remains unchanged [46].
The influence of semaphorin 3A on DCs trafficking from peripheral tissues to regional lymph nodes has been studied in details [16]. It turned out that the antigen-loaded DCs from PlexA1–/– or Nrp-1Sema– mice (in which the semaphorin 3A-binding sites are defective) accumulate in the draining lymph nodes in smaller quantities in comparison with wild-type mice. Similar effect is also observed when wild type DCs are transferred into semaphorin 3A-deficient animals. Analysis of the underlying mechanism revealed that semaphorin 3A produced by lymphatic endothelial cells is required for transmigration of DCs into efferent lymphatic vessels. This factor acts for DCs as a chemorepellent, increases their motility and speed, when it is applied against chemokine gradient in vitro, and finally enhances the process of cell transmigration across lymphatic endothelial cell monolayers [16].
In this case, semaphorin 3A signaling primarily induces the rearrangement of the actin cytoskeleton. It is known, that a cell that moves along the chemokine gradient is polarized, so that the receptor expression at the leading and trailing edges could be different. It turned out that semaphorin 3A interacts with PlexA1 at the trailing edge of DCs causing actomyosin contraction that promotes passing of the cells through narrow gaps. In the process, phosphorylation of the myosin light chains affects myosin II, while ROCK kinase and RhoA GTPase are involved in the signal transduction [16].
Semaphorin 3A also promotes migration of human DC in the absence or presence of chemokine CCL19 and it looks like chemorepulsion (away from semaphorin-containing chamber of a transwell unit) [22]. In so doing, semaphorin 3A induces a marked redistribution of F-actin into focal areas coinciding with lamellae, which is typical for directed cell migration.
Accumulating evidence indicate that PlexA1 and Nrp-1, localized on DC side of the contact area, are essential components of immunological synapse in mice [14, 35]. However, no direct interaction of semaphorin 3A with these receptors during antigen presentation stage has yet been shown. DCs from PlexA–/– mice exhibit lower potential in activation of antigen-specific or allogeneic T cells in vivo and in vitro [29, 45]. At the same time, PlexA1-deficiency does not affect antigen uptake and processing, expression of MHC class I and II molecules or costimulatory molecules CD40, CD80, and CD86 on DC surface [14, 16, 29, 45]. The presence of PlexA1 is required for the actin polarization at the interface with T cells and Rho GTPase activation in DCs; small hairpin RNA (shRNA) knockdown of PlexA1 inhibits DC-mediated antigen-specific T cell activation [14].
Similar data is obtained on human cells. Immunofluorescent and scanning microscopy demonstrate that Nrp-1 and PlexA1 localized on DCs are also functionally important components of immunological synapse [20, 34]. Adding of antibodies against PlexA1 [20] or Nrp-1 [34] into mixed allogenic cultures inhibits DC-induced proliferation of T cells.
However, the biological role of semaphorin 3A receptors, located on the membrane of DCs in the immune synapse, remains unclear. It has been suggested that PlexA1 might influence cell adhesion (or de-adhesion?), dendrite formation, and thus the ability of DCs to interact with multiple T cells [14].
Hence, DCs represent a target for semaphorin 3A at early stages of immune response, and this factor induces stimulatory effect facilitating their trafficking to lymph nodes. Contrary to T cells, that interact with semaphorin 3A via two plexin receptors, PlexA1 and PlexA4, DCs mainly engage only one of them – PlexA1.
MACROPHAGES
Semaphorin 3A, the same as for DCs, is known to be a positive regulator for macrophages. Mature macrophages synthesize this factor and possess its receptors, so they are not only a source but also a target for it.
Semaphorin 3A mRNA is usually not expressed by human peripheral blood monocytes [19, 22, 25]. However, its expression is triggered during their maturation in the presence of M-CSF [25] and is further upregulated upon activation with LPS [38]. Similar scenario is observed in mice – untreated peritoneal, splenic or bone marrow-derived macrophages poorly express semaphorin 3A mRNA, but it is substantially elevated upon activation with LPS or other TLR agonists [15].
Nrp-1 is mostly absent on human peripheral blood monocytes [22, 25, 34], and class A plexins are expressed at low levels [22, 25, 38] as it is shown by flow cytometry and RT-PCR. Receptor expression at the mRNA levels is markedly elevated (except for PlexA4) upon macrophage maturation in the presence of M-CSF [25, 38]. During further activation of human macrophages in the presence of IFN-γ, Nrp-1 mRNA level decrease similar to the expression of PlexA1 mRNA in the presence of LPS [25].
A somewhat different picture is observed in mice. Nrp-1 expression is registered already on early bone marrow-derived monocyte/macrophage precursor cells [60] and on circulating monocytes [61]. Moreover, this Nrp-1+ bone marrow monocyte/macrophage precursors also express PlexA1 and PlexA3 mRNAs [60]. During the maturation of bone marrow-derived macrophages treated with M-CSF, mRNAs of the following receptors can be detected: Nrp-1, PlexA1, A2, and A4, but not PlexA3 [18, 61].
Resident murine peritoneal macrophages express mRNA for Nrp-1 [62] and PlexA4 as well as their membrane proteins [15, 46]. With the help of flow cytometry, it is possible to detect Nrp-1 on classically (M1) and alternatively activated (M2) macrophages [63]. Activation of microglia (resident macrophages derived from newborn rat cortices) by IFN-γ in vitro or by striatal injection of LPS in vivo induces upregulation of Nrp-1 and PlexA1 [26].
Thus, the expression of semaphorin 3A receptors in rodents is observed not only in mature, but also in immature mononuclear phagocytes, and their expression is upregulated upon macrophage activation.
Semaphorin 3A is a chemoattractant for macrophages and enhances their spontaneous migration along a positive concentration gradient. This was shown in vitro with the use of transwell system for murine bone marrow-derived monocyte precursor cells [60, 64], bone marrow-derived [61], and peritoneal [17] macrophages, as well as for human CD14+ macrophages isolated from the liver of patients with hepatocellular carcinoma [65]. Semaphorin 3A also stimulates in vitro migration of bone marrow-derived murine macrophages in “wound-healing” assay (migration of cells into a scratch) [63].
Chemoattractive signals from semaphorin 3A are mediated by Nrp-1 [17, 60, 61, 64], and this effect can be abolished by administration of a selective ROCK kinase inhibitor – Y-27632 [17]. PlexA1 and PlexA4 are both responsible for semaphorin 3A-induced migration of murine macrophages, since silencing of relevant genes with small interfering RNAs (siRNA) abrogates chemotactic effect as potently as genetic deletion of Nrp-1 [61].
However, there is one study contradicting this data where it is shown that semaphorin 3A inhibits spontaneous human monocyte migration in vitro [1]. It was published in 2001 and it was the first work devoted to the influence of semaphorin 3A on migrating immune cells. In this investigation, not recombinant protein was used, but a supernatant from transfected COS7 cells that might contain additional factors besides semaphorin 3A. This suggestion is supported by several evidences, including the fact that the effect attributed to semaphorin 3A was not mediated through Nrp-1 [1].
The ability of semaphorin 3A to attract murine mononuclear phagocytes is also confirmed in vivo via the subcutaneous injection of semaphorin 3A-containing Matrigel [61] or by gene transfer of semaphorin 3A into the skeletal muscle [64] followed by quantifying the number of mononuclear cells in tissue sections. In the organism, mononuclear phagocytes can be effectively recruited into the tissues overexpressing semaphorin 3A, e.g., tumors [61] or pathologically altered retina [17].
Collectively, these findings indicate that semaphorin 3A per se is a macrophage chemoattractant. However, in combination with other stimuli in vitro, it can behave differently. For instance, semaphorin 3A stimulates migration of murine bone marrow-derived macrophages in the presence of CCL21 [62], whereas it impairs migration combined with growth factor M-CSF [18]. This effect is realized through Nrp-1 and is associated with downregulation of the M-CSF-induced RhoA signaling pathways.
Another important aspect of the effect of semaphorin 3A is the regulation of macrophage cytokine production. When macrophages are not stimulated, semaphorin 3A does not modify their production of TNF-α and IL-6 in vitro [15] as well as mRNA expression of other cytokines (IL-1β, IL-12a, IL-10, TGF-β) and chemokines [63]. However, semaphorin 3A has a pronounced and multidirectional effect on cytokine production in the case of activated macrophages.
For example, when semaphorin 3A and LPS are added simultaneously, semaphorin 3A stimulates the synthesis of TNF-α and IL-6, at that enhancing LPS effect [15]. In this case, semaphorin 3A acts via PlexA4 receptor and enhances activation of the small GTPase Rac1. Semaphorin 3A and LPS alone show elevated GTP-bound Rac1, whereas the two stimuli together cause potentiated effect. In these experiments, a suboptimal concentration of LPS was used (50 ng/ml).
In another study, a full activation of murine macrophages was applied. Cells treated with LPS/INF-γ revealed no changes of TNFα, IL-6, and IL-12a mRNA expression in response to the administration of semaphorin 3A [63]. This is in agreement with the data indicating the absence of semaphorin 3A effect on TNF-α production by human macrophages stimulated with 1 µg/ml LPS [38].
In one more work, semaphorin 3A shows different behavior and exerts inhibitory activity. Administration of semaphorin 3A to mouse bone marrow-derived macrophages causes downregulation of LPS/IFN-γ-induced production of inflammatory cytokines IL-1β, IL-6, and TNF-α [66]. The essential difference of this work is that the sequence of introduction of stimulants into cell culture was changed – macrophages were first preincubated with semaphorin 3A for 24 h and then LPS/IFN-γ was introduced [66].
It may be presumed that the sequence of introduction of stimulators in vitro plays a crucial role when these agents compete for common signaling pathways. This could partly explain controversies described above in the analysis of semaphorin 3A modulatory effects. Some other things are also very significant, like stimulator concentration defining a degree of cell activation, tissue source for cell isolation and a degree of cell maturity. To clarify this issue, it is necessary to use diverse experimental settings in a single study. At present, evaluation of the in vitro effects of semaphorin 3A on inflammatory cytokine production is extremely ambiguous, so that the criterion of their relevance can be the results obtained in vivo.
One of the key publications devoted to the study of the effect of semaphorin 3A on macrophages in the presence of other activators is the work of Wen et al. [15], where it has been shown that this factor is able to stimulate cytokine production in vivo. Semaphorin 3A injected intraperitoneally into mice with experimental polymicrobial peritonitis (partial cecal ligation and puncture) causes elevated production of inflammatory cytokines and chemokines in the peritoneal lavage fluid, that is not observed in PlexA4–/– animals. Moreover, these PlexA4–/– mice are more resistant to the development of sepsis caused by a lethal dose of LPS, and exhibit substantially lower levels of inflammatory cytokines TNF-α and IL-12p70 in the blood serum [15].
Moreover, this work established a previously unknown fact that PlexA4 is involved in transducing TLR signals. Macrophages isolated from the PlexA4–/– mice exhibit defective production of inflammatory cytokines upon activation by a spectrum of TLR agonists or bacteria [15]. Along with this, PlexA4–/– macrophages show a reduced TLR-induced Rac1 activation followed by downregulation of JNK (c-Jun N-terminal kinase) and NF-κB (nuclear factor-κB) activation. Therefore, it has been demonstrated that PlexA4 not only transduces semaphorin 3A signals that amplify LPS response, but is also involved in the novel intersection with TLR pathways unrelated to semaphorin 3A.
The data indicating that semaphorin 3A can additionally activate macrophages during the development of bacterial infection allowed to justify a new approach to treatment and prevention of sepsis in experimental animals [15] that was used later. Intravenous administration of neutralizing monoclonal anti-semaphorin 3A antibodies dose-dependently improved the survival rate in LPS-induced sepsis in mice [67]. Unexpectedly, the blood serum level of TNF-α in these animals was not decreased.
Collectively, all these findings allow us to conclude, that semaphorin 3A per se has no impact on macrophage inflammatory cytokine production, but can modulate the effects of other activating agents.
Considering that inflammatory cytokine production only partially defines activation status of macrophages, the effect of semaphorin 3A on other parameters has also been studied. Currently, several variants of macrophage activation have been described, among which there are M1 (classically activated by LPS and IFN-γ) and M2 (alternatively activated by IL-4, IL-13, or IL-10) macrophages [68]. M1 macrophages are characterized by pronounced microbicidal and tumoricidal activity, whereas M2 cells promote angiogenesis and tissue repair. The influence of semaphorin 3A on macrophage polarization has been considered in two papers.
Teng et al. have shown that pretreatment of murine bone marrow macrophages with semaphorin 3A for 24 h prohibits M1 macrophage polarization whereas it promotes M2 macrophage polarization [66]. Upon that, semaphorin 3A lowers the LPS-induced phosphorylation of STAT3 (signal transducer and activator of transcription 3), an important signaling molecule in M1 polarization.
In another work it has been investigated how already polarized M1 and M2 macrophages, treated with LPS/IFN-γ or IL-4/TGF-β, respectively, respond to semaphorin 3A. In this case, neuronal factor does not affect either cytokine and chemokine mRNA expression in mouse macrophages, or their in vitro migration [63]. However, semaphorin 3A enhances CSF1-mediated proliferation of M1 bone marrow-derived macrophages and suppresses the same activity in M2 macrophages. Semaphorin 3A also promotes the CSF1-induced Akt and MAPK phosphorylation in M1 macrophages and restricts it in M2 macrophages; these effects being mediated by Nrp-1 [63].
Thus, on the one hand, semaphorin 3A inhibits cascades typical to M1 macrophage polarization and facilitates developing of M2 phenotype [66], whereas, on the other hand, it exerts opposite effects [63]. Further research will allow for greater clarity on this issue.
Semaphorin 3A does not affect phagocytosis of the opsonized erythrocyte by human macrophages, but stimulates the development of Fas-independent apoptosis in them [25]. Semaphorin 3A is also able to induce apoptosis in the enriched culture of microglial cells (resident macrophages of the central nervous system) derived from neonatal rat cortex. Such effect is abrogated by administration of anti-Nrp-1 blocking antibodies, and is enhanced after activation of microglial cells with IFN-γ [26]. Moreover, stressed neurons produce semaphorin 3A and mediate apoptosis in activated microglia. It is suggested, that after neural injury, this reaction might suppress excessive inflammation related to activated microglia and autoprotect neurons from potentially threatening effects by secreting semaphorin 3A.
Another important area of study of macrophages is the influence of semaphorin 3A on osteoclast differentiation. These cells also belong to the mononuclear phagocyte system. One of the major factors of osteoclast differentiation is RANKL (receptor activator of nuclear factor-kB ligand), a cytokine from the TNF superfamily, that interacts with cells through its receptor RANK.
Osteoclast differentiation has been investigated on mouse bone marrow-derived monocyte/macrophage precursor cells cultivated in the presence of M-CSF and RANKL. It has been shown, that upon the addition of semaphorin 3A osteoclast differentiation is potently suppressed in vitro [66], and that this effect is mediated by Nrp-1 and PlexA1 [18]. It should be noted, that inhibition of osteoclast differentiation is valid only if semaphorin 3A is added before RANKL stimulation, but not vice versa [18]. It has been further shown, that semaphorin 3A and RANKL compete with each other for binding to PlexA1, since RANKL-mediated osteoclast differentiation requires the formation of PlexA1–TREM2–DAP12 complex.
If Nrp-1 is present on the osteoclast surface, it constitutively associates with PlexA1, which mediated semaphorin 3A signaling instead of TREM2–DAP12 signaling. In this case RANKL is not able to form the PlexA1–TREM2–DAP12 complex, and osteoclastogenesis becomes impaired. However, if RANKL is added prior to semaphorin 3A, the latter would not be able to exert its inhibitory activity, because RANKL downregulates Nrp-1 expression. As a result, PlexA1 will form the PlexA1–TREM2–DAP12 complex ensuring osteoclast differentiation [18].
The mechanism described above clearly demonstrates how a sequence of introduction of factors affects experimental data if the agents compete with each other for common receptors or signaling cascades. This may partly explain the contradicting data on semaphorin 3A-related effects in vitro.
Semaphorin 3A-induced inhibition of mouse osteoclast differentiation has also been demonstrated in vivo. In particular, semaphorin 3A-deficient animals (Sema3a–/–) or mice with functional defect of its receptor (Nrp-1Sema–) show enhanced numbers of osteoclasts and profound osteopenia [18]. Local administration of semaphorin 3A into the injured site of mice with experimental trauma (cortical bone defects induced by drill) accelerates bone regeneration; its intravenous injections to ovariectomized animals (a model of postmenopausal osteoporosis) has an osteoprotective effect. These results indicate that semaphorin 3A is a promising potential therapeutic target for bone [18] and joint [66] diseases.
Thus, the major semaphorin 3A-mediated effects on macrophages are: stimulation of migratory activity, regulation of cytokine production, and impact on macrophage polarization and differentiation.
DISCOVERY OF CHEMOREPULSION IN THE NERVOUS SYSTEM AND ITS SIGNIFICANCE FOR IMMUNOLOGY
Chemotaxis – a directed cell migration by concentration gradient of a chemokinetic agent – is one of the fundamental processes of living organisms. Currently, chemotaxis is considered as a bidirectional movement that may occur either toward the gradient of chemical agent (chemoattraction), or away from it (chemorepulsion) being regulated by a balance of chemoattractive and chemorepulsive signals in the cellular microenvironment [69].
However, it was thought for a long time that immune cells could undergo active movement only toward an agent. Despite early reports (1939) describing chemorepellent activity of aluminum silicate for human and rabbit leukocytes [70], this effect has not been practically studied.
The stimulus for such research at a new level was the discovery of chemorepulsion in the nervous system made in the late 1990s. It described, that class 3 semaphorins and some other neuronal guidance molecules could repel axon growth cones preventing developing neurons from inappropriate wiring [71].
The first immunological factors characterized as chemorepellents were two well-known chemokines SDF-1/CXCL12 and IL-8. At high concentration, SDF-1 acted not as a chemoattractant, but rather as chemorepellent for T cells [72], whereas IL-8 showed similar effect towards human neutrophils [73]. A new phenomenon that described an active movement of immune cells away from chemokinetic agent was called fugetaxis (from Greek fugere – to flee from; taxis – movement) [74]; however, this term is not widely used.
The repulsive effect of semaphorin 3A itself was demonstrated not only in the nervous system but also in the immune system. It was first discovered on human thymocytes [54], and then, on murine thymocytes [55] and DCs [16].
Chemorepulsion plays an essential role in the immune system both in health and disease. It is assumed that cell sorting of circulating cells (namely T cell exclusion) into diverse tissues may play a role in the generation of immune privileged sites of the body; additionally, chemorepellents may govern the exit of T cells from secondary lymph nodes and the thymus as well as their precursor egress from the bone marrow [74].
Class 3 semaphorins produced by thymic stromal cells are shown to be involved in sorting of precursors to the T cell lineage entering the thymus during embryonic and neonatal development [75].
It has been suggested that semaphorin 3A may exert a chemorepellent influence in the thymus promoting emigration of mature T lymphocytes to periphery in the transplantable tumor model in mice [55]. However, no data confirming this assumption was obtained, because neither semaphorin 3A nor its cognate receptor expression were found to change in the thymus during the growth of hepatoma 22a. Similarly, no difference was found in transendothelial migration of semaphorin 3A-treated thymocytes between tumor-bearing and control mice.
Chemorepulsive action of semaphorin 3A produced by many tumors may exert a significant negative effect. It may be able to promote the exit of malignant cells from the primary node and increase risk of metastasis [65]. Moreover, semaphorin 3A limits migration of tumor-specific cytotoxic T cells into the tumors, thereby contributing to cancer immune evasion [21].
Investigation of chemorepulsion not only markedly enriches our understanding of cell trafficking in the organism, but has also therapeutic applications. Chemorepellents of diverse origin may be used for the treatment of inflammatory diseases in order to exclude excessive leukocyte migration into the tissues [76] or to prevent the immune attack leading to allograft rejection [74].
CONCLUSIONS AND PERSPECTIVES
In the early 2000s, the scientific community formed the idea of “immune semaphorins” as a new group of neuronal factors with immunoregulatory properties [77]. Among these factors the most studied were semaphorins of classes 4 and 7, while semaphorin 3A was given a very modest place in this category – it was considered only as a regulator of leukocyte migration [1].
Much more detailed characteristic of semaphorin 3A appeared in 2012 [78] and since then it has been commonly accepted [3, 4, 7]. Semaphorin 3A has become a factor with dual regulation of immune response – T cell suppressor and activator of the innate immune responses [78]. Takamatsu and Kumanogoh reviewed the first works devoted to experimental application of semaphorin 3A for the treatment of immune-mediated diseases and provided a new insight into therapeutic potential of this neuronal factor.
Over the past 10 years, the study of semaphorin 3A has taken place predominantly in this direction. Multiple experiments have considered this neuronal factor, that possesses immunoregulatory and anti-angiogenic properties, as a remedy for the treatment of many human diseases including allergic, autoimmune and oncological illnesses [2-4]. Meanwhile, no new data on the role of semaphorin 3A in controlling physiological immune response has been obtained as well as no unified concept on its immunological role has yet been formulated, and even today our knowledge about this being only fragmentary.
Here, we attempted to put together all the data on immunoregulatory properties of semaphorin 3A, available over the last two decades, and discuss them from several points of view.
The main mechanism of action of semaphorin 3A on cells is actin cytoskeleton reorganization and related to it regulation of cell migration, adhesion, and cell–cell cooperation.
Another important feature of semaphorin 3A immunoregulation is the involvement of Nrp-1 and plexins in signal transduction from different factors unrelated to semaphorin 3A. By that, it may exert a modulatory effect on diverse cellular triggers including growth factors, chemokines, or bacterial products.
In some cases, antagonistic interactions between semaphorin 3A and other factors occur. In particular, semaphorin 3A suppresses the SDF-1-induced thymocyte [56] and M-CSF-induced macrophage migration [18], inhibits the proliferation of thymic epithelial cells activated by KGF or HGF [58], and negatively influences RANKL-mediated osteoclast differentiation [18]. In other cases, a synergistic interplay may arise, like enhancement of LPS-induced production of inflammatory cytokines by semaphorin 3A in mouse macrophages [15].
If we consider which functions of immune cells are affected by semaphorin 3A, it turns out that it is mainly cell migration. This factor controls migration of all cell types discussed above such as T cells [21, 23, 41], DCs [16, 22], and macrophages [17, 60, 61, 63-65]. Similar to the nervous system, this regulation can occur by chemoattraction (as for macrophages) or by chemorepulsion (as for DCs). It is suggested, that direction of movement towards or away from a chemokinetic agent might be determined by cell polarization, asymmetric distribution of surface receptors and adhesion molecules as well as by changes of intracellular signaling, including the concentration of secondary messengers such as Ca2+ and cyclic nucleotides [69, 79].
Besides migration, semaphorin 3A also affects cell adhesion and cell–cell cooperation in the immune system. It suppresses mouse thymocyte [80] and CD8+ T cell [21] adhesion to extracellular matrix, contact formation between thymocytes and thymic epithelial cells [54], as well as between DCs and T cells [20]. Moreover, semaphorin 3A inhibits interaction of T lymphocytes with tumor cells, namely T cell adhesion to them [13] and cytotoxic activity [41].
Of the other immunological functions affected by semaphorin 3A, it should be noted negative regulation of T cell [13, 19, 38] and thymocyte [57] proliferative activity as well as triggering of proapoptotic program in T cell lines [24] and human macrophages [25].
Another important issue is where and under what conditions does semaphorin 3A manifest itself in the organism. This factor is constitutively expressed in the organs of the immune system such as thymus, spleen, and lymph nodes [18, 54, 55] suggesting its essential homeostatic role. The blood serum level of semaphorin 3A in healthy individuals varies widely from 10 to 90 ng/ml or more [2]. Most authors use nearly the same concentrations (50-100 ng/ml) to study semaphorin 3A effects in vitro. It follows from this, that its serum level is sufficient for the manifestation of its biological activity. However, peripheral blood T cells and monocytes do not express semaphorin 3A receptors, therefore they are not exposed to it. Such receptors appear upon cell activation, and allow them to respond to semaphorin 3A stimuli [25, 38, 40].
At a steady state, the receptors may be found on immune cells located in tissues – on resident tissue macrophages and on a small part of T lymphocytes. Apparently, in the absence of pathology, semaphorin 3A mainly regulates tissue migration of cells and their exit from tissues into lymphatics as it was shown for DCs [16].
The role of semaphorin 3A as a regulator of cells inside the tissue is best studied in the thymus. This factor is constitutively produced by thymic epithelial cells, the targets for it being the same epithelial cells and thymocytes [54, 55, 57-59]. Semaphorin 3A controls intrathymic thymocyte migration, their adhesion/de-adhesion to/from epithelial cells, as well as proliferation of thymocytes and epithelial cells.
In pathology, the local tissue concentrations of semaphorin 3A may significantly increase. In this case, along with chemokines and growth factors, semaphorin 3A can control cell composition of the inflammatory infiltrate by selectively recruiting some cell types or restricting migration of others. For example, the elevated macrophage influx [60, 61, 63, 65] and exclusion of CD8+ T cells [21, 41] are observed in tumors with semaphorin 3A overexpression.
The effect of semaphorin 3A on different phases of the immune responses is schematically presented in Fig. 3.
At the initiation phase of the immune response, semaphorin 3A acts as a positive cue, while promoting the egress of DCs from peripheral tissues into lymphatics and their further migration to the regional lymph nodes for presentation of antigens to T lymphocytes [16].
At all subsequent stages where T cells are involved, semaphorin 3A exerts negative effects: it downregulates T cell activation and TCR-dependent signaling during antigen presentation [19, 20], inhibits the proliferation of CD4+ effector T cells and their cytokine production [38, 46], and, finally, activates Tregs, which, in turn, exert their suppressive action [38, 39]. It has been suggested, that semaphorin 3A-related effects result in the termination of the immune response, thus preventing its overactivation [19], but the precise biological role of this factor remains to be elucidated.
In some cases, semaphorin 3A-induced immunosuppression may play a positive role. Administration of exogenous semaphorin 3A is beneficial for the treatment of autoimmune and allergic diseases in experimental conditions [2, 4]. At the same time, production of this factor by growing tumors is a disadvantage for the organism (Fig. 3) – semaphorin 3A may suppress antitumor immunity inhibiting CD8+ T cell migration, adhesion, and cytotoxic activity [13, 21, 41]. In this case, scientists develop an opposite strategy aimed at blocking semaphorin 3A and its receptors in the organism [21, 41]. Clinical demands necessitate further investigation of the semaphorin 3A contribution to regulation of the T cell immune responses.
The question of the biological role of semaphorin 3A receptors (Nrp-1 and PlexA1) in immunological synapse, present on the surface of both T cells and DCs, remains unresolved [14, 19, 20, 34, 35]. Whereas the interaction of these receptors with semaphorin 3A and its negative effects on T cells have been already demonstrated, the ligands for these receptors on DCs have not yet been identified. The need for the presence of semaphorin 3A receptors on DCs to activate T cells might not be related to semaphorin 3A itself, although this hypothesis requires experimental confirmation. However, at the moment, it seems rather doubtful that in the process of intercellular interaction, a factor could suppress one cell type and simultaneously activate another.
A distinctive feature of semaphorin 3A is its ability in parallel with the inhibition of T cell immunity to have a stimulatory effect on mononuclear phagocytes. First of all, this factor acts as a chemoattractant for mouse monocytes and macrophages in vivo and in vitro [17, 60, 61, 63]. However, this experimental data cannot be directly transferred to humans, since there are interspecies differences in semaphorin 3A receptor expression. In humans, unlike mice [60, 61], virtually no semaphorin 3A receptors are identified on peripheral blood monocytes [22, 25, 34, 38], which indicates that this factor can potentially affect only activated monocytes.
Semaphorin 3A per se is not involved in stimulation of inflammatory cytokine or chemokine production by macrophages [15, 38, 63], but it can potently modulate macrophage response to other activators (e.g., LPS and IFN-γ) [15, 63, 66]. However, the results of the published works contradict each other and do not allow definitely attribute semaphorin 3A either to pro- or anti-inflammatory agents. Moreover, it is not clear at present, whether semaphorin 3A contributes to macrophage polarization toward M1 [66] or M2 phenotype [63].
Meanwhile, answers to the unsolved questions about the influence of semaphorin 3A on macrophages are important for the assessment of its role in pathogenesis of rheumatoid arthritis [66], sepsis [15], and tumor growth [60, 63], since they underlie a strategy for using semaphorin 3A as a therapeutic agent. So far, no consensus has yet been reached: some authors suggest to implement semaphorin 3A as an anti-inflammatory substance, e.g., in mouse model of rheumatoid arthritis [66], whereas the others – propose that for the same purpose this factor should be neutralized, as it was shown in an experimental model of sepsis [15, 67].
At present, there is no doubt that semaphorin 3A plays an important immunoregulatory role in health and disease, therefore allowing us to consider this factor and its receptors as promising targets for therapeutic intervention. Further research will shed light on unresolved issues and will allow to justify new approaches to the treatment of many human diseases.
Abbreviations
- DC:
-
dendritic cell
- HGF:
-
hepatocyte growth factor
- Ig:
-
immunoglobulin-like domain
- KGF:
-
keratinocyte growth factor
- LPS:
-
lipopolysaccharide
- M-CSF:
-
macrophage colony-stimulating factor
- Nrp-1:
-
neuropilin-1
- PlexA:
-
plexin A
- Sema:
-
semaphorin domain
- Tregs:
-
regulatory T cells
- TGF-β:
-
transforming growth factor β
- TLR:
-
Toll-like receptor
References
Delaire, S., Billard, C., Tordjman, R., Chedotal, A., Elhabazi, A., et al. (2001) Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration, J. Immunol., 166, 4348-4354, https://doi.org/10.4049/jimmunol.166.7.4348.
Vadasz, Z., and Toubi, E. (2018) Semaphorin3A: A potential therapeutic tool in immune-mediated disases, Eur. J. Rheumatol., 5, 58-61, https://doi.org/10.5152/eurjrheum.2017.17076.
Toledano, S., Nir-Zvi, I., Engelman, R., Kessler, O., and Neufeld, G. (2019) Class-3 semphorins and their receptors: potent multifunctional modulators of tumor progression, Int. J. Mol. Sci., 20, 556, https://doi.org/10.3390/ijms20030556.
Kalmarzi, R. N., Rajabinejad, M., and Lotfi, R. (2020) Immune semaphorins: Crucial regulatory signals and novel therapeutic targets in asthma and allergic diseases, Eur. J. Pharmacol., 881, 173209, https://doi.org/10.1016/j.ejphar.2020.173209.
Alto, L. T., and Terman, J. R. (2017) Semaphorins and their signaling mechanisms, Methods Mol. Biol., 1493, 1-25, https://doi.org/10.1007/978-1-4939-6448-2_1.
Chapoval, S. P. (2018) Neuroimmune semaphorins as costimulatory molecules and beyond, Mol. Med., 24, 13, https://doi.org/10.1186/s10020-018-0014-9.
Nakanishi, Y., Kang, S., and Kumanogoh, A. (2021) Neural guidance factors as hubs of immunometabolic cross-talk, Int. Immunol., 33, 749-754, https://doi.org/10.1093/intimm/dxab035.
Chuckran, C. A., Liu, C., Bruno, T. C., Workman, C. J., and Vignali, D. A. A. (2020) Neuropilin-1: A checkpoint target with unique implications for cancer immunology and immunotherapy, J. Immunother. Cancer, 8, e000967, https://doi.org/10.1136/jitc-2020-000967.
Niland, S., and Eble, J. A. (2020) Neuropilin: Handyman and power broker in the tumor microenvironment, Adv. Exp. Med. Biol., 1223, 31-67, https://doi.org/10.1007/978-3-030-35582-1_3.
Raper, J. A., and Kapfhammer, J. P. (1990) The enrichment of a neuronal growth cone collapsing activity from embryonic chick brain, Neuron, 4, 21-29, https://doi.org/10.1016/0896-6273(90)90440-q.
Janssen, B. J., Malinauskas, T., Weir, G. A., Cader, M. Z., Siebold, C., et al. (2012) Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex, Nat. Struct. Mol. Biol., 19, 1293-1299, https://doi.org/10.1038/nsmb.2416.
Valdembri, D., Regano, D., Maione, F., Giraudo, E., and Serini, G. (2016) Class 3 semaphorins in cardiovascular development, Cell Adhes. Migr., 10, 641-651, https://doi.org/10.1080/19336918.2016.1212805.
Catalano, A., Caprari, P., Moretti, S., Faronato, M., Tamagnone, L., et al. (2006) Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function, Blood, 107, 3321-3329, https://doi.org/10.1182/blood-2005-06-2445.
Eun, S.-Y., O’Connor, B. P., Wong, A. W., van Deventer, H. W., Taxman, D. J., et al. (2006) Cutting edge: Rho activation and actin polarization are dependent on Plexin-A1 in dendritic cells, J. Immunol., 177, 4271-4275, https://doi.org/10.4049/jimmunol.177.7.4271.
Wen, H., Lei, Y., Eun, S.-Y., and Ting, J. P. (2010) Plexin-A4 semaphorin 3A signaling is required for Toll-like receptor and sepsis-induced cytokine storm, J. Exp. Med., 207, 2943-2957, https://doi.org/10.1084/jem.20101138.
Takamatsu, H., Takegahara, N., Nakagawa, Y., Tomura, M., Taniguchi, M., et al. (2010) Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II, Nat. Immunol., 11, 594-600, https://doi.org/10.1038/ni.1885.
Dejda, A., Mawambo, G., Cerani, A., Miloudi, K., Shao, Z., et al. (2014) Neuropilin-1 mediates myeloid cell chemoattraction and influences retinal neuroimmune crosstalk, J. Clin. Invest., 124, 4807-4822, https://doi.org/10.1172/JCI76492.
Hayashi, M., Nakashima, T., Taniguchi, M., Kodama, T., Kumanogoh, A., et al. (2012) Osteoprotection by semaphorin 3A, Nature, 485, 69-74, https://doi.org/10.1038/nature11000.
Lepelletier, Y., Moura, I. C., Hadj-Slimane, R., Renand, A., Fiorentino, S., et al. (2006) Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization, Eur. J. Immunol., 36, 1782-1793, https://doi.org/10.1002/eji.200535601.
Tran-Van, H., Avota, E., Bortlein, C., Mueller, N., and Schneider-Schaulies, S. (2011) Measles virus modulates dendritic cell/T-cell communication at the level of plexinA1/neuropilin-1 recruitment and activity, Eur. J. Immunol., 41, 151-163, https://doi.org/10.1002/eji.201040847.
Barnkob, M., Michaels, Y. S., André, V., Macklin, P. S., Gileadi, U., et al. (2019) Semaphorin 3A induces cytoskeletal paralysis in tumor-specific CD8+ T cells, BioRxiv, https://doi.org/10.1101/849083.
Curreli, S., Wong, B. S., Latinovic, O., Konstantopoulos, K., and Stamatos, N. M. (2016) Class 3 semaphorins induce F-actin reorganization in human dendritic cells: role in cell migration, J. Leukoc. Biol., 100, 1323-1334, https://doi.org/10.1189/jlb.2A1114-534R.
Vincent, P., Collette, Y., Marignier, R., Vuaillat, C., Rogemond, V., et al. (2005) A role for the neuronal protein collapsin response mediator protein 2 in T lymphocyte polarization and migration, J. Immunol., 175, 7650-7660, https://doi.org/10.4049/jimmunol.175.11.7650.
Moretti, S., Procopio, A., Lazzarini, R., Rippo, M. R., Testa, R., et al. (2008) Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by promoting Fas translocation into lipid rafts, Blood, 111, 2290-2299, https://doi.org/10.1182/blood-2007-06-096529.
Ji, J. D., Park-Min, K. H., and Ivashkiv, L. B. (2009) Expression and function of semaphorin 3A and its receptors in human monocyte-derived macrophages, Hum. Immunol., 70, 211-217, https://doi.org/10.1016/j.humimm.2009.01.026.
Majed, H. H., Chandran, S., Niclou, S. P., Nicholas, R. S., Wilkins, A., et al. (2006) A novel role for Sema3A in neuroprotection from injury mediated by activated microglia, J. Neurosci., 26, 1730-1738, https://doi.org/10.1523/jneurosci.0702-05.2006.
Battin, C., Linhares, A. D. S., Paster, W., Isenman, D. E., Wahrmann, M., et al. (2019) Neuropilin-1 acts as a receptor for complement split products, Front. Immunol., 10, 2209, https://doi.org/10.3389/fimmu.2019.02209.
Daly, J. L., Simonetti, B., Klein, K., Chen, K. E., Williamson, M. K., et al. (2020) Neuropilin-1 is a host factor for SARS-CoV-2 infection, Science, 370, 861-865, https://doi.org/10.1126/science.abd3072.
Takegahara, N., Takamatsu, H., Toyofuku, T., Tsujimura, T., Okuno, T., et al. (2006) Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis, Nat. Cell Biol., 8, 615-622, https://doi.org/10.1038/ncb1416.
Fujisawa, H., Ohta, K., Kameyama, T., and Murakami, Y. (1997) Function of a cell adhesion molecule, plexin, in neuron network formation, Dev. Neurosci., 9, 101-105, https://doi.org/10.1159/000111192.
Takagi, S., Kasuya, Y., Shimizu, M., Matsuura, T., Tsuboi, M., et al. (1995) Expression of a cell adhesion molecule, neuropilin, in the developing chick nervous system, Dev. Biol., 170, 207-222, https://doi.org/10.1006/dbio.1995.1208.
Valdembri, D., Caswell, P. T., Anderson, K. I., Schwarz, J. P., Konig, I., et al. (2009) Neuropilin-1/GIPC1 signaling regulates α5β1 integrin traffic and function in endothelial cells, PLoS Biol., 7, e1000025, https://doi.org/10.1371/journal.pbio.1000025.
Rutto, K. V., Kudryavtsev, I. V., and Kisseleva, E. P. (2018) Adhesion of thymocytes to the thymic epithelial cells and participation of neuropilin-1 and plexin A1 in the adhesion, Cell Tissue Biol., 12, 373-381, https://doi.org/10.1134/S1990519X18050073.
Tordjman, R., Lepelletier, Y., Lemarchandel, V., Cambot, M., Gaulard, P., et al. (2002) A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response, Nat. Immunol., 3, 477-482, https://doi.org/10.1038/ni789.
Sarris, M., Andersen, K. G., Randow, F., Mayr, L., and Betz, A. G. (2008) Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition, Immunity, 28, 402-413, https://doi.org/10.1016/j.immuni.2008.01.012.
Delgoffe, G. M., Woo, S.-R., Turnis, M. E., Gravano, D. M., Guy, C., et al. (2013) Stability and function of regulatory T cells is maintained by a neuropilin-1–semaphorin-4a axis, Nature, 501, 252-256, https://doi.org/10.1038/nature12428.
Glinka, Y., and Prud’homme, G. J. (2008) Neuropilin-1 is a receptor for transforming growth factor β-1, activates its latent form, and promotes regulatory T cell activity, J. Leukoc. Biol., 84, 302-310, https://doi.org/10.1189/jlb.0208090.
Catalano, A. (2010) The neuroimmune semaphorin-3A reduces inflammation and progression of experimental autoimmune arthritis, J. Immunol., 185, 6373-6383, https://doi.org/10.4049/jimmunol.0903527.
Cozacov, R., Halasz, K., Haj, T., and Vadasz, Z. (2017) Semaphorin 3A: Is a key player in the pathogenesis of asthma, Clin. Immunol., 184, 70-72, https://doi.org/10.1016/j.clim.2017.05.011.
Milpied, P., Renand, A., Bruneau, J., Mendes-da-Cruz, D. A., Jacquelin, S., et al. (2009) Neuropilin-1 is not a marker of human Foxp3+ Treg, Eur. J. Immunol., 39, 1466-1471, https://doi.org/10.1002/eji.200839040.
Leclerc, M., Voilin, E., Gros, G., Corgnac, S., de Montpréville, V., et al. (2019) Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by Neuropilin-1, Nat. Commun., 10, 3345, https://doi.org/10.1038/s41467-019-11280-z.
Chaudhary, B., and Elkord, E. (2015) Novel expression of Neuropilin 1 on human tumor-infiltrating lymphocytes in colorectal cancer liver metastases, Expert Opin. Ther. Targets, 19, 147-161, https://doi.org/10.1517/14728222.2014.977784.
Takagawa, S., Nakamura, F., Kumagai, K., Nagashima, Y., Goshima, Y., et al. (2013) Decreased Semaphorin3A expression correlates with disease activity and histological features of rheumatoid arthritis, BMC Musculoskelet. Disord., 14, 40, https://doi.org/10.1186/1471-2474-14-40.
Corbel, C., Lemarchandel, V., Thomas-Vaslin, V., Pelus, A. S., Agboton, C., et al. (2007) Neuropilin 1 and CD25 co-regulation during early murine thymic differentiation, Dev. Comp. Immunol., 31, 1082-1094, https://doi.org/10.1016/j.dci.2007.01.009.
Wong, A. W., Brickey, W. J., Taxman, D. J., van Deventer, H. W., Reed, W., et al. (2003) CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions, Nat. Immunol., 4, 891-898, https://doi.org/10.1038/ni960.
Yamamoto, M., Suzuki, K., Okuno, T., Ogata, T., Takegahara, N., et al. (2008) Plexin-A4 negatively regulates T lymphocyte responses, Int. Immunol., 20, 413-420, https://doi.org/10.1093/intimm/dxn006.
Battaglia, A., Buzzonetti, A., Monego, G., Peri, L., Ferrandina, G., et al. (2008) Neuropilin-1 expression identifies a subset of regulatory T cells in human lymph nodes that is modulated by preoperative chemoradiation therapy in cervical cancer, Immunology, 123, 129-138, https://doi.org/10.1111/j.1365-2567.2007.02737.x.
Jung, K., Kim, J.-A., Kim, Y.-J., Lee, H. W., Kim, C.-H., et al. (2020) A Neuropilin-1 antagonist exerts antitumor immunity by inhibiting the suppressive function of intratumoral regulatory T cells, Cancer Immunol. Res., 8, 46-56, https://doi.org/10.1158/2326-6066.CIR-19-0143.
E, X. Q., Meng, H. X., Cao, Y., Zhang, S. Q., Bi, Z. G., et al. (2012) Distribution of regulatory T cells and interaction with dendritic cells in the synovium of rheumatoid arthritis, Scand. J. Rheumatol., 41, 413-420, https://doi.org/10.3109/03009742.2012.696135.
Smyth, L. J., Starkey, C., Vestbo, J., and Singh, D. (2007) CD4-regulatory cells in COPD patients, Chest, 132, 156-163, https://doi.org/10.1378/chest.07-0083.
Bruder, D., Probst-Kepper, M., Westendorf, A. M., Geffers, R., Beissert, S., et al. (2004) Neuropilin-1: A surface marker of regulatory T cells, Eur. J. Immunol., 34, 623-630, https://doi.org/10.1002/eji.200324799.
Weiss, J. M., Bilate, A. M., Gobert, M., Ding, Y., Curotto de Lafaille, M. A., et al. (2012) Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells, J. Exp. Med., 209, 1723-1742, https://doi.org/10.1084/jem.20120914.
Solomon, B. D., Mueller, C., Chae, W. J., Alabanza, L. M., and Bynoe, M. S. (2011) Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis, Proc. Natl. Acad. Sci. USA, 108, 2040-2045, https://doi.org/10.1073/pnas.1008721108.
Lepelletier, Y., Smaniotto, S., Hadj-Slimane, R., Villa-Verde, D. M., Nogueira, A. C., et al. (2007) Control of human thymocyte migration by Neuropilin-1/Semaphorin-3A-mediated interactions, Proc. Natl. Acad. Sci. USA, 104, 5545-5550, https://doi.org/10.1073/pnas.0700705104.
Kisseleva, E. P., Lyamina, I. V., Tzvirkun, S. A., Rutto, K. V., Kudryavtsev, I. V., et al. (2013) Investigation of the role of semaphoring 3A in the thymus at normal conditions and tumor growth [in Russian], Med. Acad. J., 13, 42-48, https://doi.org/10.17816/MAJ13442-48.
Garcia, F., Lepelletier, Y., Smaniotto, S., Hadj-Slimane, R., Dardenne, M., et al. (2012) Inhibitory effect of semaphorin-3A, a known axon guidance molecule in the human thymocyte migration induced by CXCL12, J. Leukoc. Biol., 91, 7-13, https://doi.org/10.1189/jlb.0111031.
Rutto, K. V., Lyudyno, V. I., Kudryavtsev, I. V., and Kiseleva, E. P. (2020) Semaphorin-3A inhibits proliferation, but does not affect apoptosis of mouse thymocytes in vitro, Bull. Exp. Biol. Med., 168, 352-355, https://doi.org/10.1007/s10517-020-04707-x.
Rutto, K. V., Ovsyukov, K. S., Kudryavtsev, I. V., and Kiseleva, E. P. (2019) Semaphorin 3A negatively affects proliferation of mouse thymus epithelial cell in vitro, Bull. Exp. Biol. Med., 166, 339-343, https://doi.org/10.1007/s10517-019-04346-x.
Mendes-da-Cruz, D. A., Lepelletier, Y., Brignier, A. C., Smaniotto, S., et al. (2009) Neuropilins, semaphorins, and their role in thymocyte development, Ann. N. Y. Acad. Sci., 1153, 20-28, https://doi.org/10.1111/j.1749-6632.2008.03980.x.
Carrer, A., Moimas, S., Zacchigna, S., Pattarini, L., Zentilin, L., et al. (2012) Neuropilin-1 identifies a subset of bone marrow Gr1 monocytes that can induce tumor vessel normalization and inhibit tumor growth, Cancer Res., 72, 6371-6381, https://doi.org/10.1158/0008-5472.CAN-12-0762.
Casazza, A., Laoui, D., Wenes, M., Rizzolio, S., Bassani, N., et al. (2013) Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity, Cancer Cell, 24, 695-709, https://doi.org/10.1016/j.ccr.2013.11.007.
Stepanova, O. I., Krylov, A. V., Lioudyno, V. I., and Kisseleva, E. P. (2007) Gene expression for VEGF-A, VEGF-C, and their receptors in murine lymphocytes and macrophages, Biochemistry (Moscow), 72, 1194-1198, https://doi.org/10.1134/s0006297907110041.
Wallerius, M., Wallmann, T., Bartish, M., Ostling, J., Mezheyeuski, A., et al. (2016) Guidance molecule SEMA3A restricts tumor growth by differentially regulating the proliferation of tumor-associated macrophages, Cancer Res., 76, 3166-3178, https://doi.org/10.1158/0008-5472.CAN-15-2596.
Zacchigna, S., Pattarini, L., Zentilin, L., Moimas, S., Carrer, A., et al. (2008) Bone marrow cells recruited through the neuropilin-1 receptor promote arterial formation at the sites of adult neoangiogenesis in mice, J. Clin. Invest., 118, 2062-2075, https://doi.org/10.1172/JCI32832.
Hu, Z.-Q., Zhou, S.-L., Zhou, Z.-J., Luo, C.-B., Chen, E.-B., et al. (2016) Overexpression of semaphoring 3A promotes tumor progression and predicts poor prognosis in hepatocellular carcinoma after curative resection, Oncotarget, 7, 51733-51746, https://doi.org/10.18632/oncotarget.10104.
Teng, Y., Yin, Z., Li, J., Li, K., Li, X., and Zhang, Y. (2017) Adenovirus-mediated delivery of Sema3A alleviates rheumatoid arthritis in a serum-transfer induced mouse model, Oncotarget, 8, 66270-66280, https://doi.org/10.18632/oncotarget.19915.
Yamashita, N., Jitsuki-Takahashi, A., Ogawara, M., Ohkubo, W., Araki, T., et al. (2015) Anti-Semaphorin 3A neutralization monoclonal antibody prevents sepsis development in lipopolysaccharide-treated mice, Int. Immunol., 27, 459-466, https://doi.org/10.1093/intimm/dxv014.
Martinez, F. O., and Gordon, S. (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment, F1000Prime Rep., 6, 13, https://doi.org/10.12703/P6-13.
Huttenlocher, A., and Poznansky, M. C. (2008) Reverse leukocyte migration can be attractive or repulsive, Trends Cell Biol., 18, 298-306, https://doi.org/10.1016/j.tcb.2008.04.001.
McCutcheon, M., Coman, D. R., and Dixon, H. M. (1939) Negative chemotropism in leukocytes, Arch. Pathol., 17, 61-68.
Polleux, F., Giger, R. J., Ginty, D. D., Kolodkin, A. L., and Ghosh, A. (1998) Patterning of cortical efferent projections by semaphorin-neuropilin interactions, Science, 282, 1904-1906, https://doi.org/10.1126/science.282.5395.1904.
Poznansky, M. C., Olszak, I. T., Foxall, R., Evans, R. H., Luster, A. D., et al. (2000) Active movement of T cells away from a chemokine, Nat. Med., 6, 543-548, https://doi.org/10.1038/75022.
Tharp, W. G., Yadav, R., Irimia, D., Upadhyaya, A., Samadani, A., et al. (2006) Neutrophil chemorepulsion in defined interleukin-8 gradients in vitro and in vivo, J. Leukoc. Biol., 79, 539-554, https://doi.org/10.1189/jlb.0905516.
Vianello, F., Olszak, I. T., and Poznansky, M. C. (2005) Fugetaxis: Active movement of leukocytes away from chemokinetic gradient, J. Mol. Med., 83, 752-763, https://doi.org/10.1007/s00109-005-0675-z.
Takahashi, K., Ishida, M., Hirokawa, K., and Takahashi, H. (2008) Expression of the semaphorins Sema 3D and Sema 3F in the developing parathyroid and thymus, Dev. Dyn., 237, 1699-1708, https://doi.org/10.1002/dvdy.21556.
Herlihy, S. E., Brown, M. L., Pilling, D., Weeks, B. R., Myers, L. K., et al. (2015) Role of the neutrophil chemorepellent soluble dipeptidyl peptidase IV in decreasing inflammation in a murine model of arthritis, Arthritis Rheumatol., 67, 2634-2638, https://doi.org/10.1002/art.39250.
Kumanogoh, A., and Kikutani, H. (2003) Immune semaphorins: a new area of semaphorin research, J. Cell Sci., 116 (Pt 17), 3463-3470, https://doi.org/10.1242/jcs.00674.
Takamatsu, H., and Kumanogoh, A. (2012) Diverse roles for semaphorin-plexin signaling in the immune system, Trends Immunol., 33, 127-135, https://doi.org/10.1016/j.it.2012.01.008.
Tojima, T., Hines, J. H., Henley, J. R., and Kamiguchi, H. (2011) Second messengers and membrane trafficking direct and organize growth cone steering, Nat. Rev. Neurosci., 12, 191-203, https://doi.org/10.1038/nrn2996.
Lins, M. P., Silva, E. C. O., Silva, G. R., Souza, S. T., Medeiros, N. C., et al. (2018) Association between biomechanical alterations and migratory ability of semaphorin-3A-treated thymocytes, Biochim. Biophys. Acta Gen. Subj., 1862, 816-824, https://doi.org/10.1016/j.bbagen.2018.01.001.
Funding
This work was carried out within the framework of the planned research project of the Federal State Budgetary Scientific Institution “IEM” FGWG-2022-0005 (no. 122020300186-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kiseleva, E.P., Rutto, K.V. Semaphorin 3A in the Immune System: Twenty Years of Study. Biochemistry Moscow 87, 640–657 (2022). https://doi.org/10.1134/S0006297922070069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297922070069