Abstract
Early-life stress is a risk factor for the development of behavioral and cognitive disorders in humans and animals. Such stressful situations include social isolation in early postnatal ontogenesis. Behavioral and cognitive impairments associated with neuroplastic changes in brain structures. We have found that after ten weeks of social isolation, male Wistar rats show behavioral abnormalities and cognitive deficit, accompanied by an increase in the relative expression of gene encoding serine protease prolyl endopeptidase (PREP, EC 3.4.21.26) in the brain frontal cortex. The present study aimed to assess synaptophysin (SYP), brain-derived neurotrophic factor precursor (proBDNF), and PREP expression using Western blot in the brain structures – the hippocampus, frontal cortex, and striatum of the rats subjected to prolonged social isolation compared with group-housed animals. Twenty Wistar rats were used for this study (10 males and 10 females). Experimental animals (5 males and 5 females) were kept one per cage for nine months, starting from the age of one month. Ten-month-old socially isolated rats showed memory deficit in passive avoidance paradigm and Morris Water Maze and reactivity to novelty reduction. We used monoclonal antibodies for the Western blot analysis of the expression of SYP, proBDNF, and PREP in the rat brain structures. Social isolation caused a proBDNF expression reduction in the frontal cortex in females and a reduction in PREP expression in the striatum in males. These data suppose that neurotrophic factors and PREP are involved in the mechanisms of behavioral and cognitive impairments observed in the rats subjected to prolonged social isolation with an early life onset.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Stress at the early stages of ontogenesis is a risk factor of behavioral and cognitive impairments, as well as some somatic disorders [1-4]. A type of stress is the early-life social isolation (deprivation), accompanied by the limited sensory stimulation and insufficient formation of social, cognitive, and verbal skills.
Social isolation (SI) is an important stressor in both humans and social animals (e.g., rodents) [5, 6]. Reported that, socially isolated rats develop excessive aggressiveness, impaired motivation, hyperactive phenotype, and cognitive deficit [7]. It is believed these behavioral changes are determined by alterations in the neuroplasticity of brain structures [8] and disruption of genome epigenetic programming at an early age [9-11].
Changes in the synaptic plasticity associated primarily with the activity of neurons, are a fundamental component of neuroplasticity. In turn these changes are mediated through the regulation of synaptic proteins [12]. The glycoprotein synaptophysin (SYP) is the main integral membrane protein of small synaptic vesicles (vesicles that do not contain peptides). SYP is involved in the formation of synaptic vesicles, release of neurotransmitters, and synaptogenesis [13, 14]. The data on the impact of various types of chronic stress (immobilization, unpredictable stress, maternal separation in rats) on the SYP expression are contradictory [15-21], which could be due to the nature of stress and specifics of stress response in animals of different strains, sex, and age. The data on the effects of SI are scarce and inconsistent as well. Chronic 8-week-long SI did not affect SYP expression in the hippocampus (HPC) and hypothalamus of adult female Sprague–Dawley rats [22], while in male Lister hooded rats, 8-week-long SI started from weaning led to the decrease in the SYP content in the dentate gyrus of the HPC [23]. Two-week-long SI of adult Sprague–Dawley male rats caused a decrease in the SYP expression and synaptic plasticity in the HPC [24]. To the best of our knowledge, the data on the SYP expression in rat brain structures under prolonged SI are absent.
Another well-known biomarker and regulator of synaptic and neuronal plasticity is a brain-derived neurotrophic factor (BDNF), the most abundant neurotrophin in the mammalian brain, which is considered one of the most important stress responses mediators [25, 26]. BDNF regulates neuronal survival and differentiation and modulates various synaptic functions (e.g., facilitates long-term potentiation and structural increase of dendritic spines) [27]. BDNF is synthesized as a pre-proBDNF precursor in the endoplasmic reticulum, then transported to the Golgi apparatus, where the proBDNF isoform is produced. Mature BDNF (mBDNF) is formed from proBDNF via cleavage of the pro-domain sequence [28, 29]. The regulatory effects of proBDNF and BDNF on the synaptic plasticity are different and often opposite. It is assumed that proBDNF could be a key regulator of synaptic plasticity and formation of neural circuits in adolescence, and these effects are preserved in adult animals [27]. The disturbance in the balance between proBDNF and mBDNF due to the presumed inhibition of precursor conversion into mBDNF was observed in the HPC of rats in the two-hit rat stress model (chronic neonatal maternal separation and administration of corticosteroids to adult animals) [30] and in the prenatal stress model [31]. Early SI starting from weaning and continued for two weeks (postnatal days 21-34) resulted in decreased pre-pulse inhibition in the sensorimotor reactivity test and epigenetic modification of BDNF (upregulation of BDNF expression in the medial prefrontal cortex and decrease of its expression in the HPC) in male Sprague–Dawley rats [32]. The data on whether the level of BDNF isoforms changes during the long-term SI, and, if yes, then how, are not currently available.
Our studies in the rat model of SI started from weaning and continued for two months demonstrated that male Wistar rats exhibited more pronounced aggressive behavior, decrease of pre-pulse inhibition, and cognitive deficit manifested as learning impairments (evaluated by the decrease in the acoustic startle response amplitude) accompanied by the upregulated expression of the prep gene encoding serine proteinase prolyl endopeptidase (PREP, EC 3.4.21.26) in the frontal cortex (FC) [33]. PREP is a pleiotropic protease [34] involved in numerous processes, including learning, memory, and neuroplasticity, likely via interactions with other proteins [35]. Age-related increase in the PREP activity in the FC, hypothalamus, nucleus accumbens, and striatum (STR) of male rats and in HPC and STR of female rats was demonstrated in the model of the mixed anxiety-depressive state induced by the dipeptidyl peptidase IV (EC 3.4.14.5) inhibitor in the early postnatal period [36]. These rats demonstrated slower learning in the active avoidance test than the control group animals [37]. The anxiety-depressive state induced by another inhibitor of dipeptidyl peptidase IV was accompanied by the upregulation of the prep gene expression in the STR [38]. An increase in the PREP activity in the FC and HPC of male Wistar rats was observed in the model of experimental retrograde amnesia induced by the m-cholinoblocker scopolamine (muscarinic receptor antagonist) or by the maximal electroshock [39]. Recently, we demonstrated that the long-term SI started from weaning and continued for nine months resulted in the disruption of cognitive abilities of both male and female Wistar rats in the passive avoidance test, as well as spatial memory impairments that were more pronounced in females [40].
The objective of this study was to assess the levels of SYP, proBDNF, and PREP proteins in the brain structures (hippocampus, frontal cortex, and striatum) of rats subjected to the long-term SI in comparison with the group-housed animals using the Western blotting technique.
MATERIALS AND METHODS
Modeling of SI in rats and behavioral tests. Expression of SYP, proBDNF, and PREP protein was assessed in 20 Wistar rats (ten males, ten females). The rats were bred and housed at the Animal Facility of the Institute of General Pathology and Pathophysiology (System Merkuryi, registration no. RU 1487336). Animals were selected from the larger groups of male and female Wistar rats that were housed either individually (SI) or in groups (control) for nine months and have passed behavioral testing. The mass of each tissue sample of the brain structures from the selected animals was at least 100 mg, which allowed us to perform Western blotting within the method sensitivity range. The results of behavior evaluation for the large groups of rats in the open field test (OF), passive avoidance test (PA), and Morris water maze (MWM) test have been published previously [40]. In this study, animals were subjected to the same behavioral tests and then assessed for protein expression in the brain in order to evaluate whether the behavior of animals in the small sample was consistent with the behavioral changes previously observed in the large groups of animals. Starting from the age of 1 month and until the end of the experiment at the age of ten months, ten animals (“control, males”, n = 5; “control, females” group, n = 5) were housed in groups of 4-5 animals per cage (cage size, 57.0 × 37.0 × 19.0 cm), while 10 other rats (“isolation, males”, n = 5; “isolation, females”, n = 5) were housed individually (cage size, 36.5 × 20.5 × 14.0 cm). The animals were kept under standard conditions at the natural light/dark cycle and ad libitum access to food (Laboratorkorm Ltd., Russia) and water.
At the end of the experiment, the locomotor activity and memory of the animals were assessed in the automated OF, classic OF, PA, and MWM test as described in detail in the study by Krupina et al. [40].
Automated OF (evaluation of locomotor activity). In the automated OF (aOF), the locomotor activity (presented in arbitrary cm) was evaluated in an open arena (48 × 48 × 21 cm) with transparent walls (Opto-Varimex, Columbus Instruments, USA) by the number of interruptions of infrared rays within 10 min under mild illumination (17 lux).
Classic OF (evaluation of locomotor activity and reactivity to novelty). In the classic OF (cOF), the locomotor activity was evaluated in a round white arena (diameter, 120 cm) with non-transparent walls (height, 28 cm). The arena was divided into 20 squares. The locomotor activity was determined as the number of crossed squares within 3-min observation under bright illumination (500-510 lux in the center and 400-410 lux at the arena periphery). After 3 min, white light was replaced with a low-intensity red light (40 W above the arena center). The reactivity to novelty was determined by the increase in the locomotor activity in response to changes in the environment (illumination switch as a novelty factor) [41]. The ratio of the distance covered during the 4th minute of observation under the red light and the distance covered during the 1st minute under the white light was calculated.
Passive avoidance test. PA was conducted in a chamber divided by a guillotine door into two sections: “safe” bright-lit compartment and “dangerous” dark compartment with an electrified floor. The chamber was placed into a soundproof system (Multi Conditioning System, TSE System, Germany). On the first day, the rats get habituated to the chamber, in which they explored the illuminated (156 lux) compartment for 60 s and then were allowed to enter the dark compartment for 120 s. The following day, 24 h later, we subjected rats to an acquisition trial. The rat was placed into the illuminated compartment for 5 s, and then the door to the dark compartment opened automatically. One second after the animal entered the dark compartment, the door closed; 5 s later, an electric shock (0.5 mA, 1 Hz, for 5 s) was delivered through the grid floor and 30 s later the rat was returned to the cage. The retention of the passive avoidance response was tested 24 h (Test 1) and one week (Test 2) after the acquisition trial. The latency to enter the dark compartment (s) was registered in all tests. The testing protocol was the same as the protocol used for the acquisition trial, except that no electric shock was applied. The maximum possible time to enter the dark compartment was 300 s.
Morris water maze test (evaluation of spatial memory). MWM was a round pool (diameter, 160 cm) filled with water to the depth of 30 cm; water temperature was 25 ± 1°C. Rat behavior was monitored via an automated video tracking system VideoMot2 (TSE System). The pool was virtually divided into four quadrants. During the acquisition sessions, a transparent platform (diameter, 14 cm) was placed in the target quadrant (the same in all experiments) 1.5 cm below the water surface, and visual cues were placed outside of the pool. The acquisition sessions were started when the rats were 5.5-month-old. For four consecutive days, the rats were given an opportunity to find the hidden platform, while the start quadrant was alternated in a pseudorandom order. The maximum duration of the trial was 2 min. The developed skill was tested in a 2-min probe 24 h after the last acquisition session; for the probe the platform was removed from the pool. Memory retention was tested again under the same conditions 4 months after the acquisition sessions (in 9.5-month-old rats). The day following this probe, the rats were given two reminder trials (maximum trial duration, 2 min) to reach the platform that was placed in the same quadrant. A day later the platform was removed and the probe was performed again. In each probe, the latency to reach the platform site or the area around the platform site (10 cm from the rim of the platform) and relative time spent in the border area (20% of the pool radius) were recorded. Here, we present the data for the last probe for all animal groups. At the age of ten months, all rats were sacrificed by decapitation.
Sample preparation. Rat brain was removed immediately after decapitation and placed into cold saline. HPC, FC, and STR were isolated on ice, frozen in liquid nitrogen, and stored at –80°C in a Sanio MDF-193 freezer (Sanio, Japan). Analyzed samples were disintegrated by grinding in liquid nitrogen and lyzed to prepare cytosolic extracts in the hypotonic buffer I [20 mM Tris (Bio-Rad, USA), pH 7.5, 1 mM EDTA (Sigma-Aldrich, USA), 1 mM DTT (Bio-Rad), and 10 µl/ml protease inhibitor cocktail (Sigma-Aldrich)]. Lysis with hypotonic buffer I was performed for 5 min at 4°C; the supernatant was separated by centrifugation at 1000 rpm for 5 min at 4°C; (Eppendorf, Germany). Sludge lysis with buffer II [60 mM HEPES, 150 mM NaCl, 2 mM EGTA, 1% (w/v) Triton X-100, 10% (v/v) glycerol, 1 mM DTT, and 10 µl/ml protease inhibitor cocktail (Sigma-Aldrich)] was carried out for 25 min at 4°C; the samples were centrifuged at 13,000 rpm for 25 min at 4°C. The resulting cytosolic extracts were mixed with the loading buffer [0.5 M Tris-HCl, pH 6.8, 0.08 g/ml SDS, 5 mg/ml DTT, 0.2 mg/ml bromophenol blue, 40% (v/v) glycerol] at a 1 : 3 ratio, incubated for 5 min at 95°C, and stored at –80°C.
Assessment of SYP, proBDNF, and PREP expression by Western blotting. Total protein concentration in the samples was determined using Bradford protein assay [42]. The samples (140 mg of total protein) were loaded onto 8% (w/v) polyacrylamide gel, fractionated by electrophoresis at 120 V for 90 min, and then transferred onto nitrocellulose membrane (Bio-Rad) at 100 V for 60 min. The membrane was blocked in PBS containing 5% fat-free milk (Bio-Rad), 0.1% (v/v) Tween 20, and 0.02% sodium azide for 1 h at 4°C. Simultaneously, primary mouse antibodies against SYP (2) (sc-136271, 38-48 kDa), proBDNF (5H8; c-65514, 32 kDa), PREP (C-12; sc-365416, 80 kDa), and β-actin (C4; sc-47778, 43 kDa) (Santa Cruz Biotechnology, USA) were incubated at 4°C in PBS with 5% fat-free milk, 0.1% (v/v) Tween 20, and 0.02% sodium azide. Next, the membrane was incubated with the primary antibodies for 15 h at 4°C, washed, and incubated for 60 min with the secondary antibody (goat anti-mouse IgG-HRP; ab6789, Abcam, USA) at 4°C. Proteins were visualized with the ECL reagent (Pierce Biotechnology, USA) by placing the membrane under a photo film (Kodak, USA) for 10 min. Densitometry was performed using the Adobe Photoshop 7.0 software (Adobe Systems, USA); the results were presented in relative densitometry units (RDU). Protein load was verified by staining on the nitrocellulose membrane with Ponceau S and measuring expression of β-actin (reference protein).
Statistical data processing was performed with the Statistica 12.0 software. Since the Shapiro–Wilk test did not confirm the normal distribution of empirical data in the small groups, the non-parametric Mann–Whitney U-test was used to evaluate the differences between two independent groups; the accepted significance level was 5%. To account for multiple comparisons, the critical p-value (pcr) was calculated according to the FDR control method [43]. The pcr < p < 0.05 value was considered as a pronounced trend; the 0.05 < p < 0.06 value – as a trend. The effect size (ES) was calculated using the η2 (proportion of variance attributed to one or more effects) and Cohen’s d (dCohen) indices [44]. The ES was interpreted as follows: dCohen, 0.20-0.40 – low; 0.50-0.70 – medium, from 0.80 and above – high; η2, 0.010-0.039 – low; 0.060-0.110 – medium; 0.140-0.200 – high [45].
RESULTS
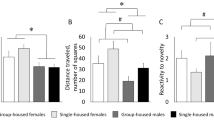
Behavioral tests. Automated OF. The locomotor activity of all female rats housed in groups and individually was higher than that of all male rats (U = 18.0; p = 0.015; η2 = 0.293; dCohen = 1.286) (Fig. 1a); however, the locomotor activity of single-housed rats (males + females) did not differ from the locomotor activity of group-housed rats (U = 34.0; p = 0.247). No statistically significant difference for this parameter was revealed between rats of the same sex housed individually or in groups.
Classic OF. The locomotor activity of female rats (all groups) in this test was higher than the locomotor activity of all male rats (U = 23.5; p = 0.043; η2 = 0.201; dCohen = 1.002); moreover, the difference was determined by the activity of group-housed and not single-housed rats [U = 0.0; p = 0.008 (pkp = 0.013); η2 = 0.682; dCohen = 2.928] (Fig. 1b). The locomotor activity of rats housed individually (males + females) did not differ from the locomotor activity of group-housed animals (males + females) (U = 29.0; p = 0.123). However, the locomotor activity of single-housed males was statistically significantly higher than the activity of group-housed males [U = 1.0; p = 0.016 (pcr = 0.025); η2 = 0.577; dCohen = 2.336]. Single-housed rats (males + females) exhibited lower reactivity to novelty in comparison with the group-housed rats (U = 18.0; p = 0.015; η2 = 0.293; dCohen = 1.286) (Fig. 1c). No difference in the reactivity to novelty was observed between male and female rats.
Locomotor activity, learning, and memory in rats subjected to long-term SI and control animals (C) at the age of 9-10 months (n = 5 in each group) in the OF (a-c), PA (d-f), and MWM (g-i) test. a) Total locomotor activity within 10 min in aOF: b) total locomotor activity within 3 min in cOF; c, reactivity to novelty in cOF; d-f) latency to enter the dark compartment in PA (d) in the acquisition trial; (e) in the memory retention test 24 h after the acquisition trial (Test 1), and (f) one week after the first test (Test 2); g) relative time spent in the border area of the pool in MWM test; h) latency to reach the platform site in MWM; i) latency to reach the area around the platform site in MWM; * p < 0.05, statistically significant difference between all males and all females; # p < 0.05, statistically significant difference between all single-housed rats and all group-housed rats; ^ p < 0.05, in comparison with the group-housed male rats; + pcr < p < 0.05, in comparison with the group-housed animals of the same sex; & 0.05 < p < 0.06 , in comparison with the group-housed female rats (Mann–Whitney U-test with correction for multiple comparisons using the FDR control technique).
Passive avoidance test. In the acquisition trial, the latency to enter the dark compartment did not differ between the groups (Fig. 1d). One day after the acquisition trial, the latency to enter the dark compartment was significantly lower in the single-housed rats (males + females) in comparison with the group-housed animals (males + females): U = 23.0; p = 0.043; η2 = 0.208; dCohen = 1.026) (Fig. 1e). In the memory retention test one week after the first test, the latency to enter the dark compartment was also shorter in the single-housed rats (males + females) than in the group-housed animals (males + females): U = 2.5; p < 0.001; η2 = 0.645; dCohen = 2.694) (Fig. 1f). The trend for shortening the latency to enter the dark compartment was revealed in both single-housed male rats in comparison with the group-housed animals [U = 0.0; p = 0.016 (pcr = 0.013), η2 = 0.682; dCohen = 2.928] and in single-housed female rats in comparison with the group-housed females [U = 2.0; p = 0.032 (pcr = 0.025); η2 = 0.481; dCohen = 1.926]. In all memory retention tests, no difference was observed in the latency to enter the dark compartment for rats of the different sex housed under the same conditions.
Morris water maze test. Single-housed rats (males + females) did not differ from the group-housed rats in the relative time spent in the border area of the pool: U = 31.0; p = 0.165. No statistically significant differences were observed for this parameter between all female rats (housed in groups and individually) and all male rats: U = 27.0; p = 0.089. However, a trend for an increase in this indicator was found in the single-housed female rats in comparison with the group-housed females (Fig. 1g): U = 3.0; p = 0.056; η2 = 0.394; dCohen = 1.612.
There were no differences in the latency to reach the platform site between all rats, both individually or group-housed, as well as between all males and all females (Fig. 1h).
Single-housed rats (males + females) reached the area around the platform later than the group-housed rats (males + females): U = 22.0; p = 0.035; η2 = 0.224; dCohen = 1.075. The data presented in Fig. 1i demonstrate that the latency to reach the area around the platform was significantly longer for some female rats housed individually. However, statistical analysis revealed only a trend for the increase in this parameter in single-housed female rats in comparison with the group-housed females: U = 3.0; p = 0.056; η2 = 0.394; dCohen = 1.612.
Expression of SYP, proBDNF, and PREP proteins in the brain structures. In the HPC, expression of SYP, proBDNF, and PREP did not differ in the individually and group-housed rats of the same sex [Fig. 2a (1 and 2), b, and c; Fig. 3, a (1 and 2), b and c; Fig. 4, a (1 and 2), b and c]. The observed difference between the SYP content in the single- and group-housed male rats (Fig. 2b) did not reach statistical significance: U = 5.0; p = 0.151; η2 = 0.245; dCohen = 1.141.
a) Western blot of SYP expression in the brain: HPC of male (1) and female (2) rats; FC of male (3) and female (4) rats, STR of male (5) and female (6) rats. b-g) SYP expression in the brain structures of rats individually housed for 9 months (SI) and control group-housed rats (C): HPC of male (b) and female (c) rats; FC of male (d) and female (e) rats; STR of male (f) and female (g) rats. In the box and whiskers plot: bottom of the box, Q1 (first quartile); top of the box, Q3 (third quartile), median is marked with black square, Q2 (second quartile); box height, interquartile range. RDU, relative densitometry unit; 0.05 < # p < 0.06 comparison with the group-housed rats of the same sex (two-tailed Mann–Whitney U-test).
In the FC, we also observed a trend for the decrease in the SYP expression in individually housed male rats compared with the male rats housed in groups [Fig. 2, a (3), d]: U = 3.0; p = 0.056; η2 = 0.394; dCohen = 1.612. Expression of proBDNF in the single-housed female rats was significantly decreased vs. the group-housed females [Fig. 3, a (4), e]: U = 2.0; p = 0.032; η2 = 0.481; dCohen = 1.926. PREP expression in the single-housed animals (both males and females) did not differ from its expression in the group-housed rats [Fig. 4, a (3 and 4), d and e].
a) Western blot of proBDNF expression in the brain: a) HPC of male (1) and female (2) rats; FC of male (3) and female (4) rats, STR of male (5) and female (6) rats. b-g) proBDNF expression in the brain structures of rats individually housed for 9 months (SI) and control group-housed rats (C): HPC of male (b) and female (c) rats; FC of male (d) and female (e) rats; STR of male (f) and female (g) rats. RDU, relative densitometry unit; * p < 0.05, comparison with the group-housed rats of the same sex (two-tailed Mann–Whitney U-test).
a) Western blot of PREP expression in the brain: a) HPC of male (1) and female (2) rats; FC of male (3) and female (4) rats, STR of male (5) and female (6) rats. b-g) PREP expression in the brain structures of rats individually housed for 9 months (SI) and control group-housed rats (C): HPC of male (b) and female (c) rats; FC of male (d) and female (e) rats; STR of male (f) and female (g) rats. RDU, relative densitometry unit; * p < 0.05 and # p < 0.06, comparison with the group-housed rats of the same sex (two-tailed Mann–Whitney U-test).
In the STR, PREP expression in individually housed male rats was significantly lower than in the group-housed males [Fig. 4, a (5), f]: U = 0.0; p = 0.029; η2 = 0.667; dCohen = 2.828. A trend for the reduced expression of PREP was observed in the individually housed female rats [Fig. 4, a (6), g]: U = 1.0; p = 0.057; η2 = 0.51; dCohen = 2.042. No differences were revealed between the contents of SYP and proBDNF in the STR of the individually and group-housed rats of the same sex.
No statistically significant differences in the expression of β-actin in the brain structures were detected in the single-housed and group-housed animals (analysis of β-actin in the brain structures by Western blotting and examples of developed films and membranes stained for total protein with molecular weight markers are presented in Figs. S1 and S2 in the Supplement).
DISCUSSION
We found that the locomotor activity of female rats in the automated and classic OF tests was higher than the activity of male rats in all investigated animals (n = 20). The reactivity to novelty in the classic OF in the rats housed individually for ~8 months (SI) was reduced irrespectively of the animal sex. These results are in general agreement with the data obtained in large groups of Wistar rats (n = 69) subjected to SI of the same duration [40]. Evaluation of the cognitive functions in the PA and MWM test also indicated similar changes in both small and large groups of animals: SI impaired passive avoidance and spatial memory (based on the latency to reach the area around the platform; Fig. S3 in Supplement) irrespectively of the animal sex. These data suggest that the smaller samples of rats (for which expression of proteins-markers of neuroplasticity and PREP in the brain structures was evaluated) rather well represent the initial large group of rats subjected to the long-term SI judging by behavioral impairments.
In the present study, the decrease in the proBDNF level was found only for the single-housed female rats and only in the FC. Evaluation of the spatial memory in the MWM test indicated that the memory of female rats subjected to chronic SI stress was worse than that of the group-housed animals and single-housed male rats (Fig. 1, g and i). This suggestion is in agreement with the conclusion of our recent study conducted in a large group of animals that the prolonged SI started at an early age caused more pronounced cognitive impairments in females than in male rats (according to the MWM test), which indicates higher susceptibility of female rats to the long-term SI stress [40]. Marco et al. [46] showed that impaired novel object recognition observed in female rats (but not in male rats) subjected to the stress of one-day-long maternal deprivation in the early postnatal period was not accompanied by the differences in the expression of BDNF in the FC and HPC of the rats of both sexes in adolescence. However, it should be taken into account that in our study, we evaluated expression not of the mature form of BDNF, but of its precursor that regulates synaptic plasticity and formation of neural circuits in adolescence [27]. The results of the current study support the hypothesis that the diverse functions of neurotrophins could be modulated in part by the regulated release of their mature and pro-isoforms in the nervous system [47]. It is likely that the decrease in the proBDNF level in the FC of the 10-months-old female rats reflected changes in the neuroplasticity that have occurred during the early stages of development when the animals were already subjected to the SI. If the proBDNF/mBDNF imbalance accompanies these changes, it could be one of the mechanisms underlying a higher susceptibility of female rats to the long-term SI stress starting at an early age. There are indications that the functioning of the proBDNF–p75NTR signaling cascade in the neurons of adult mice inhibits the activity of the pyramidal cells in the layer V of the HPC entorhinal cortex, the excitation of which is a key component of the working and spatial memory [48]. Blocking proBDNF by antibodies enhanced the excitability of pyramidal neurons in the HPC.
Several studies showed that cognitive impairments caused in rats by the SI continuing for 4 to 8 weeks at different periods of postnatal ontogenesis were accompanied by the enhanced expression of apoptosis markers [49-51]. Experiments in cultured sympathetic neurons showed that proBDNF could exert the pro-apoptotic effect in the case of complex formation between p75NTR (neurotrophin receptor) and NTSR3/sortilin receptor (non-G-protein coupled receptor of neurotensin-3) [47]. The decrease in the proBDNF expression in the FC of female rats with the SI-induced memory deficit may be an adaptive response to the pro-apoptotic activity of this protein.
We also found a trend for a decrease in the SYP content in the FC of individually housed male rats. Carvalho-Netto et al. [52] showed that the SYP immunoreactivity in the prefrontal cortex of Sprague–Dawley rats not subjected to stress was lower in male than in female rats. The authors considered this fact a confirmation of sex differences in the physiological organization of the presynaptic innervation in rats; however, the 14-day-long chronic unpredictable stress failed to change the immunoreactivity of this protein in both male and female rats. In our study, chronic stress was of fundamentally different nature and significantly more prolonged, but no statistically significant differences in the SYP expression were observed as well. It is noteworthy that changes in the expression levels of one of neuroplasticity markers, e.g., BDNF (in our study, its immature form proBDNF) or SYP, could occur without changes in the levels of another marker in the same brain structure [53].
Unexpectedly, we detected no changes in the content of neuroplasticity markers in the HPC after the long-term (9 months) SI stress, while such changes have been repeatedly reported for other types of stress and shorter SI (2- and 8-week-long) [16, 20, 21, 23, 24]. However, no changes in the SYP level in the HPC were observed in adolescent rats after acute stress of maternal deprivation and in adult female rats after 8-week-long SI [22, 46]. One of the principal explanations for the absence of changes in the levels of neuroplasticity markers in the HPC in our study could be the long period of SI during which the animals have adapted to the neuroplasticity changes that occurred at an early age.
To the best of our knowledge, we have shown for the first time that the long-term SI caused a decrease in the PREP level in the STR of male rats. No statistically significant changes in the PREP expression in this brain structure were found in female rats, although, the reactivity to novelty and learning in the PA were impaired in the animals of both sexes. Cognitive deficit is presumably associated with an increase in the PREP activity in the brain, and some PREP inhibitors exhibit the anti-amnesic properties [39, 54]. Currently, there is no answer to whether the decrease in the PREP content in the STR of male rats subjected to the long-term SI stress is accompanied by changes in the expression of the prep gene and the activity of the encoded enzyme. The significance of the decrease in the PREP level could be elucidated only by comparing these data. However, the fact that expression of the prep gene following 10-week-long SI started from weaning was altered in the FC of male rats [33], while the level of the PREP protein following 9-month-long SI was changed in the STR deserves special attention. Both FC and STR are involved in learning [55]. Still, it has to be investigated how PREP participates in these processes in rats of different sexes and whether its role changes with the increasing duration of SI.
Is there any association between the observed changes in the expression of proBDNF and PREP in the brain upon long-term SI stress? At first sight, the answer is rather negative, and the revealed changes likely reflect the induction of some independent mechanisms, such as changes in the brain neurotrophic system or in the activity of one of proline-specific peptidases. However, this association cannot be ruled out entirely. As mentioned above, chronic SI promotes apoptosis in the brain structures [49-51], which could be triggered by proBDNF under certain conditions [47]. We were unable to find any published direct evidence on the PREP association with apoptosis. The studies using enzyme inhibitors produced contradictory data. Thus, Fiedorowicz et al. [56] demonstrated that PREP inhibition did not prevent the neurotoxin-initiated death of granular neurons in the dentate gyrus of the HPC. On the contrary, other authors have shown that PREP inhibitors efficiently prevented age-associated apoptosis of cultured cortical neurons and cerebellar granular cells, as well as delayed neuronal death in the HPC induced by transient ischemia (cited from [57]). The authors of studies demonstrating the protective role of PREP inhibitors hypothesized that this effect could be mediated by neuropeptides – enzyme substrates involved in the anti-apoptotic mechanisms. In this context, neurotensin is of special attention. Neurotensin is a neuropeptide with promnesic properties; it is expressed in the STR [58, 59] and cleaved by PREP [60, 61]. It exhibits the antiapoptotic effect in the CNS by interacting with the cognate receptors, NTSR3/sortilin, in particular [62]. NTSR3/sortilin can form heterodimers (protein complexes) with the neurotrophin receptors, including p75NTR, which, as has been mentioned above, triggers the proBDNF-induced neuronal death. Neurotensin counteracts the proneurotrophin-induced apoptosis by competing for the NTSR3/sortilin binding site and acting as a competitive inhibitor [47, 63]. If we accept that the long-term SI is accompanied by the apoptosis development in rats (yet to be demonstrated for this model), we can suggest that SI initiates different protective mechanisms in animals depending on sex. For example, it downregulates PREP expression in the STR of male rats, which leads to the increase in the content of the anti-apoptotic substrate neurotensin, while in female rats, SI decreases the expression of proBDNF in the FC, resulting in the attenuation of its proapoptotic effects. However, this hypothesis requires further investigation and validation.
Our study has limitations, which have to be considered when interpreting the results. First of all, they are the small sample size and the absence of assessment of the expression of genes encoding the proteins of interest. We did not examine the activity of the proline-specific protease PREP; the neuroplasticity dynamics and changes in the PREP activity during the long-term SI. Given this, we believe this study to be a pilot project. Continuing this investigation will produce new data on the effects of the long-term SI on the brain neurotrophic system and the role of PREP in the sex-specific behavioral and cognitive disorders in rats.
Abbreviations
- BDNF:
-
brain-derived neurotrophic factor
- FC:
-
frontal cortex
- HPC:
-
hippocampus
- MWM:
-
Morris water maze
- OF:
-
open field test
- PA:
-
passive avoidance test
- PREP:
-
prolyl endopeptidase
- SI:
-
social isolation
- STR:
-
striatum
- SYP:
-
synaptophysin
References
Walker, A. J., Kim, Y., Price, J. B., Kale, R. P., McGillivray, J. A., et al. (2014) Stress, inflammation, and cellular vulnerability during early stages of affective disorders: biomarker strategies and opportunities for prevention and intervention, Front. Psychiatry, 5, 34, https://doi.org/10.3389/fpsyt.2014.00034.
Vargas, J., Junco, M., Gomez, C., and Lajud, N. (2016) Early life stress increases metabolic risk, HPA axis reactivity, and depressive-like behavior when combined with postweaning social isolation in rats, PLoS One, 11, e0162665, https://doi.org/10.1371/journal.pone.0162665.
Friedler, B., Crapser, J., and McCullough, L. (2015) One is the deadliest number: the detrimental effects of social isolation on cerebrovascular diseases and cognition, Acta Neuropathol., 129, 493-509, https://doi.org/10.1007/s00401-014-1377-9.
Duffy, K. A., McLaughlin, K. A., and Green, P. A. (2018) Early life adversity and health-risk behaviors: proposed psychological and neural mechanisms, Ann. N. Y. Acad. Sci., 1428, 151-169, https://doi.org/10.1111/nyas.13928.
Mumtaz, F., Khan, M. I., Zubair, M., and Dehpour, A. R. (2018) Neurobiology and consequences of social isolation stress in animal model – a comprehensive review, Biomed. Pharmacother., 105, 1205-1222, https://doi.org/10.1016/j.biopha.2018.05.086.
Schweinfurth, M. K. (2020) The social life of Norway rats (Rattus norvegicus), Elife, 9, e54020, https://doi.org/10.7554/eLife.54020.
Fone, K. C., and Porkess, M. V. (2008) Behavioural and neurochemical effects of post-weaning social isolation in rodents – relevance to developmental neuropsychiatric disorders, Neurosci. Biobehav. Rev., 32, 1087-1102, https://doi.org/10.1016/j.neubiorev.2008.03.003.17.
Liu, N., Wang, Y., An, A. Y., Banker, C., Qian, Y.-H., and O’Donnell, J. M. (2020) Single housing-induced effects on cognitive impairment and depression-like behavior in male and female mice involve neuroplasticity-related signaling, Eur. J. Neurosci., 52, 2694-2704, https://doi.org/10.1111/ejn.14565.
Siuda, D., Wu, Z., Chen, Y., Guo, L., Linke, M., et al. (2014) Social isolation-induced epigenetic changes in midbrain of adult mice, J. Physiol. Pharmacol., 65, 247-255.
Murgatroyd, C., Patchev, A., Wu, Y., Micale, V., Bockmühl, Y., et al. (2009) Dynamic DNA methylation programs persistent adverse effects of early-life stress, Nat. Neurosci., 12, 1559-1566, https://doi.org/10.1038/nn.2436.
Gapp, K., von Ziegler, L., Tweedie-Cullen, R. Y., and Mansuy, I. M. (2014) Early life epigenetic programming and transmission of stress-induced traits in mammals, BioEssays, 36, 491-502, https://doi.org/10.1002/bies.201300116.
Gulyaeva, N. V. (2017) Molecular mechanisms of neuroplasticity: an expanding universe, Biochemistry (Moscow), 82, 237-242, https://doi.org/10.1134/S0006297917030014.
Korzhevskii, D. E., Petrova, E. S., Kirik, O. V., Beznin, G. V., and Sukhrukova, E. G. (2010) Neural markers used for investigation of differentiation of stem cells, Geny Kletki, 5, 57-63.
Kolos, E. A., Grigoriev, I. P., and Korzhevskii, D. E. (2015) Marker of synaptic contacts – synaptophisin, Morfologiya, 147, 79-83.
Hao, Y., Shabanpoor, A., and Metz, G. A. (2017) Stress and corticosterone alter synaptic plasticity in a rat model of Parkinson’s disease, Neurosci. Lett., 651, 79-87, https://doi.org/10.1016/j.neulet.2017.04.063.
Xu, H., He, J., Richardson, J. S., and Li, X. M. (2004) The response of synaptophysin and microtubule-associated protein 1 to restraint stress in rat hippocampus and its modulation by venlafaxine, J. Neurochem., 91, 1380-1388, https://doi.org/10.1111/j.1471-4159.2004.02827.x.
Gemmel, M., Kokras, N., Dalla, C., and Pawluski, J. L. (2018) Perinatal fluoxetine prevents the effect of pre-gestational maternal stress on 5-HT in the PFC, but maternal stress has enduring effects on mPFC synaptic structure in offspring, Neuropharmacology, 128, 68-180, https://doi.org/10.1016/j.neuropharm.2017.10.009.
Zhang, L., Luo, J., Zhang, M., Yao, W., Ma, X., and Yu, S. Y. (2014) Effects of curcumin on chronic, unpredictable, mild, stress-induced depressive-like behaviour and structural plasticity in the lateral amygdala of rats, Int. J. Neuropsychopharmacology, 17, 793-806, https://doi.org/10.1017/S1461145713001661.
Hescham, S., Grace, L., Kellaway, L. A., Bugarith, K., and Russell, V. A. (2009) Effect of exercise on synaptophysin and calcium/calmodulin-dependent protein kinase levels in prefrontal cortex and hippocampus of a rat model of developmental stress, Metab. Brain Dis., 24, 701-709, https://doi.org/10.1007/s11011-009-9165-2.
Andersen, S. L., and Teicher, M. H. (2004) Delayed effects of early stress on hippocampal development, Neuropsychopharmacology, 29, 1988-1993, https://doi.org/10.1038/sj.npp.1300528.
Dandi, Ε., Kalamari, A., Touloumi, O., Lagoudaki, R., Nousiopoulou, E., et al. (2018) Beneficial effects of environmental enrichment on behavior, stress reactivity and synaptophysin/BDNF expression in hippocampus following early life stress, Int. J. Dev. Neurosci., 67, 19-32, https://doi.org/10.1016/j.ijdevneu.2018.03.003.
Ramos-Ortolaza, D. L., Doreste-Mendez, R. J., Alvarado-Torres, J. K., and Torres-Reveron, A. (2017) Ovarian hormones modify anxiety behavior and glucocorticoid receptors after chronic social isolation stress, Behav. Brain Res., 328, 115-122, https://doi.org/10.1016/j.bbr.2017.04.016.
Varty, G. B., Marsden, C. A., and Higgins, G. A. (1999) Reduced synaptophysin immunoreactivity in the dentate gyrus of prepulse inhibition-impaired isolation-reared rats, Brain Res., 824, 197-203, https://doi.org/10.1016/s0006-8993(99)01173-7.
Das, S. K., Baitharu, I., Barhwal, K., Hota, S. K., and Singh, S. B. (2016) Early mood behavioral changes following exposure to monotonous environment during isolation stress is associated with altered hippocampal synaptic plasticity in male rats, Neurosci. Lett., 612, 231-237, https://doi.org/10.1016/j.neulet.2015.12.038.
Bondar, N. P., and Merkulova, T. I. (2016) Brain-derived neurotrophic factor and early-life stress: Multifaceted interplay, J. Biosci., 41, 751-758, https://doi.org/10.1007/s12038-016-9648-3.
Paltsyn, A. A. (2019) Neurotrophic brain factor – BDNF, Patogenez, 17, 83-88, https://doi.org/10.25557/2310-0435.2019.03.83-88.
Hempstead, B. L. (2015) Brain-derived neurotrophic factor: three ligands, many actions, Trans. Am. Clin. Climatol. Assoc., 126, 9-19.
Giacobbo, L. B., Doorduin, J., Klein, H. C., Dierckx, R. A. J. O., Bromberg, E., and de Vries, E. F. J. (2019) Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation, Mol. Neurobiol., 56, 3295-3312, https://doi.org/10.1007/s12035-018-1283-6.
Wang, M., Xie, Y., and Qin, D. (2021) Proteolytic cleavage of proBDNF to mBDNF in neuropsychiatric and neurodegenerative diseases, Brain Res. Bull., 166, 172-184, https://doi.org/10.1016/j.brainresbull.2020.11.005.
Hill, R. A., Klug, M., Kiss Von Soly, S., Binder, M. D., Hannan, A. J., and van den Buuse, M. (2014) Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling, Hippocampus, 24, 1197-1211, https://doi.org/10.1002/hipo.22302.
Yeh, C. M., Huang, C. C., and Hsu, K. S. (2012) Prenatal stress alters hippocampal synaptic plasticity in young rat offspring through preventing the proteolytic conversion of pro-brain-derived neurotrophic factor (BDNF) to mature BDNF, J. Physiol., 590, 991-1010, https://doi.org/10.1113/jphysiol.2011.222042.
Li, M., Du, W., Shao, F., and Wang, W. (2016) Cognitive dysfunction and epigenetic alterations of the BDNF gene are induced by social isolation during early adolescence, Behav. Brain Res., 313, 177-183, https://doi.org/10.1016/j.bbr.2016.07.025.
Zubkov, E. A., Zorkina, Ya. A., Orshanskaya, E. V., Khlebnikova, N. N., Krupina, N. A., and Chekhnin, V. P. (2019) Post-weaning social isolation disturbs gene expression in rat brain structures, Bull. Exper. Biol. Med., 166, 364-368, https://doi.org/10.1007/s10517-019-04351-0.
García-Horsman, J. A. (2020) The role of prolyl oligopeptidase, understanding the puzzle, Ann. Transl. Med., 8, 983, https://doi.org/10.21037/atm-20-3412.
Männistö, P. T., and García-Horsman, J. A. (2017) Mechanism of action of prolyl oligopeptidase (PREP) in degenerative brain diseases: has peptidase activity only a modulatory role on the interactions of PREP with proteins? Front. Aging Neurosci., 9, 27, https://doi.org/10.3389/fnagi.2017.00027.
Kushnareva, E. Yu., Krupina, N. A., Khlebnikova, N. N., Zolotov, N. N., and Kryzhanovskii, G. N. (2011) Activities of proline-specific peptidases in brain structures of rats with experimental anxiety-depressive state caused by administration dipeptidyl peptidase IV inhibitor in the early postnatal period, Bull. Exp. Biol. Med., 151, 675-679, https://doi.org/10.1007/s10517-011-1413-x.
Khlebnikova, N. N., Krupina, N. A., Kushnareva, E. Yu., and Orlova, I. N. (2015) Differences in active avoidance conditioning in male and female rats with experimental anxiety-depressive disorder, Bull. Exp. Biol. Med., 159, 337-340, https://doi.org/10.1007/s10517-015-2956-z.
Zubkov, E. A., Zorkina, Y. A., Orshanskaya, E. V., Khlebnikova, N. N., Krupina, N. A., and Chekhonin, V. P. (2017) Changes in gene expression profiles in adult rat brain after neonatal action of dipeptidyl peptidase-IV inhibitors, Neuropsychobiology, 76, 89-99, https://doi.org/10.1159/000488367.
Nazarova, G. A., Zolotov, N. N., Krupina, N. A., Kraineva, V. A., Garibova, T. L., and Voronina, T. A. (2007) Changes in proline-specific peptidase activity in experimental model of retrograde amnesia, Eksperim. klinich. farmakol., 70, 6-8, https://doi.org/10.30906/0869-2092-2007-70-6-6-8.
Krupina, N. A., Shirenova, S. D., and Khlebnikova, N. N. (2020) Prolonged social isolation, started early in life, impairs cognitive abilities in rats depending on sex, Brain Sci., 10, 799, https://doi.org/10.3390/brainsci10110799.
Hall, F. S., Humby, T., Wilkinson, L., and Robbins, T. (1997) The effects of isolation-rearing of rats on behavioural responses to food and environmental novelty, Physiol. Behav., 62, 281-290, https://doi.org/10.1016/S0031-9384(97)00115-7.
Bradford, M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 72, 248-254, https://doi.org/10.1006/abio.1976.9999.
Benjamini, Y., and Hochberg, Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing, J. R. Stat. Soc. Ser. B, 57, 289-300, https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
Psychometrica: Computation of effect sizes. 11. Effect size calculator for non-parametric tests: Mann-Whitney-U, Wilcoxon-W and Kruskal-Wallis-H, URL: https://www.psychometrica.de/effect_size.html.
Cohen, J. (1988) Statistical Power Analysis for the Behavioral Sciences, Edn. 2, Hillsdale, Erlbaum.
Marco, E. M., Valero, M., de la Serna, O., Aisa, B., Borcel, E., et al. (2013) Maternal deprivation effects on brain plasticity and recognition memory in adolescent male and female rats, Neuropharmacology, 68, 223-231, https://doi.org/10.1016/j.neuropharm.2012.08.014.
Teng, H. K., Teng, K. K., Lee, R., Wright, S., Tevar, S., et al. (2005) ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin, J. Neurosci., 25, 5455-5463, https://doi.org/10.1523/JNEUROSCI.5123-04.2005.
Gibon, J., Buckley, S. M., Unsain, N., Kaartinen, V., Seguela, P., and Barker, P. A. (2015) proBDNF and p75NTR control excitability and persistent firing of cortical pyramidal neurons, J. Neurosci., 35, 9741-9753, https://doi.org/10.1523/jneurosci.4655-14.2015.
Park, S.-S, Park, H. S., Kim, T. W., and Lee, S. J. (2020) Effects of swimming exercise on social isolation-induced memory impairment and apoptosis in old rats, J. Exerc. Rehabil., 16, 234-241, https://doi.org/10.12965/jer.2040366.183.
Park, H.-S., Kim, T.-W., Park, S.-S., Lee, S.-J. (2020) Swimming exercise ameliorates mood disorder and memory impairment by enhancing neurogenesis, serotonin expression, and inhibiting apoptosis in social isolation rats during adolescence, J. Exerc. Rehabil., 16, 132-140, https://doi.org/10.12965/jer.2040216.108.
Kim, T.-W., Park, S.-S., Shin, M.-S., Park, H.-S., and Baek, S.-S. (2020) Treadmill exercise ameliorates social isolation-induced memory impairment by enhancing silent information regulator-1 expression in rats, J. Exerc. Rehabil., 16, 227-233, https://doi.org/10.12965/jer.2040400.200.
Carvalho-Netto, E. F., Myers, B., Jones, K., Solomon, M. B., and Herman, J. P. (2011) Sex differences in synaptic plasticity in stress-responsive brain regions following chronic variable stress, Physiol. Behav., 104, 242-247, https://doi.org/10.1016/j.physbeh.2011.01.024.
Yau, S. Y., Li, A., Zhang, E. D., Christie, B. R., Xu, A., et al. (2014) Sustained running in rats administered corticosterone prevents the development of depressive behaviors and enhances hippocampal neurogenesis and synaptic plasticity without increasing neurotrophic factor levels, Cell Transplant., 23, 481-492, https://doi.org/10.3727/096368914X678490.
Babkova, K., Korabecny, J., Soukup, O., Nepovimova, E., Jun, D., and Kuca, K. (2017) Prolyl oligopeptidase and its role in the organism: attention to the most promising and clinically relevant inhibitors, Future Med. Chem., 9, 1015-1038, https://doi.org/10.4155/fmc-2017-0030.
Yavas, E., Gonzalez, S., and Fanselow, M. S. (2019) Interactions between the hippocampus, prefrontal cortex, and amygdala support complex learning and memory, F1000Res., 31, 8, https://doi.org/10.12688/f1000research.19317.1.
Fiedorowicz, A., Figiel, I., Kamińska, B., Zaremba, M., Wilk, S., and Oderfeld-Nowak, B. (2001) Dentate granule neuron apoptosis and glia activation in murine hippocampus induced by trimethyltin exposure, Brain Res., 912, 116-127, https://doi.org/10.1016/s0006-8993(01)02675-0.
Bär, J. W., Rahfeld, J.-U., Schulz, I., Gans, K., Ruiz-Carrillo, D., et al. (2006) Prolyl endopeptidase cleaves the apoptosis rescue peptide humanin and exhibits an unknown post-cysteine cleavage specificity, Adv. Exp. Med. Biol., 575, 103-108, https://doi.org/10.1007/0-387-32824-6_11.
Zahm, D. S. (1987) Neurotensin-immunoreactive neurons in the ventral striatum of the adult rat: ventromedial caudate-putamen, nucleus accumbens and olfactory tubercle, Neurosci. Lett., 81, 41-47, https://doi.org/10.1016/0304-3940(87)90337-5.
Liu, Q., Hazan, A., Grinman, E., and Angulo, J. A. (2017) Pharmacological activation of the neurotensin receptor 1 abrogates the methamphetamine-induced striatal apoptosis in the mouse brain, Brain Res., 1659, 148-155, https://doi.org/10.1016/j.brainres.2017.01.029.
Jalkanen, A. J., Puttonen, K. A., Venäläinen, J. I., Sinervä, V., Mannila, A., et al. (2006) Beneficial effect of prolyl oligopeptidase inhibition on spatial memory in young but not in old scopolamine-treated rats, Basic Clin. Pharmacol. Toxicol., 100, 132-138, https://doi.org/10.1111/j.1742-7843.2006.00021.x.
Peltonen, I., Myöhänen, T. T., and Männistö, P. T. (2012) Different interactions of prolyl oligopeptidase and neurotensin in dopaminergic function of the rat nigrostriatal and mesolimbic pathways, Neurochem. Res., 37, 2033-2041, https://doi.org/10.1007/s11064-012-0825-y.
Devader, C., Béraud-Dufour, S., Coppola, T., and Mazella, J. (2013) The anti-apoptotic role of neurotensin, Cells, 2, 124-135, https://doi.org/10.3390/cells2010124.
Nykjaer, A., and Willnow, T. E. (2012) Sortilin: a receptor to regulate neuronal viability and function, Trends Neurosci., 35, 261270, https://doi.org/10.1016/j.tins.2012.01.003.
Acknowledgments
The authors express their gratitude to Dr. Yulia Igorevna Kirova for advice and help with Western blotting.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 20-315-90110) and the State Assignment of the Federal State Budgetary Scientific Institution “Research Institute of General Pathology and Pathophysiology (reg. No. AAAA-A19-119100790089-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. All manipulations on animals were performed in accordance with EU Directive 2010/63/EU, under the control of the Ethics Committee of the Institute of General Pathology and Pathophysiology (project approval protocol No. 6 of 23.11.2018; the final approval protocol No. 3 of 16.06.2020). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
Rights and permissions
Open access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shirenova, S.D., Khlebnikova, N.N. & Krupina, N.A. Long-Term Social Isolation Reduces Expression of the BDNF Precursor and Prolyl Endopeptidase in the Rat Brain. Biochemistry Moscow 86, 704–715 (2021). https://doi.org/10.1134/S0006297921060080
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297921060080