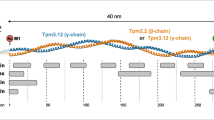
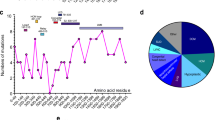
Abstract
The review is devoted to tropomyosin (Tpm)–actin-binding protein, which plays a crucial role in the regulation of contraction of skeletal and cardiac muscles. Special attention is paid to myopathies and cardiomyopathies–severe hereditary diseases of skeletal and cardiac muscles associated with point mutations in Tpm genes. The current views on the molecular mechanisms of these diseases and the effects of such mutations on the Tpm structure and functions are considered in detail. Besides, some part of the review is devoted to analysis of the properties of Tpm homodimers and heterodimers with myopathic substitutions of amino acid residues in only one of the two chains of the Tpm dimeric molecule.
Similar content being viewed by others
Abbreviations
- Tpm:
-
tropomyosin
References
Gunning, P., O’Neill, G., and Hardeman, E. (2008) Tropomyosin-based regulation of the actin cytoskeleton in time and space, Physiol. Rev., 88, 1–35.
Gunning, P., Gordon, M., Wade, R., Gahlmann, R., Lin, C. S., and Hardeman, E. (1990) Differential control of tropomyosin mRNA levels during myogenesis suggests the existence of an isoform competition-autoregulatory compensation control mechanism, Dev. Biol., 138, 443–453.
Weinberger, R. P., Henke, R. C., Tolhurst, O., Jeffrey, P. L., and Gunning, P. (1993) Induction of neuron-specific tropomyosin mRNAs by nerve growth factor is dependent on morphological differentiation, J. Cell Biol., 120, 205–215.
Grieshaber, N. A., Ko, C., Grieshaber, S. S., Ji, I., and Ji, T. H. (2003) Follicle-stimulating hormone-responsive cytoskeletal genes in rat granulosa cells: class I beta-tubulin, tropomyosin-4, and kinesin heavy chain, Endocrinology, 144, 29–39.
Schevzov, G., Vrhovski, B., Bryce, N. S., Elmir, S., Qiu, M. R., O’Neill, G., N. Yang, N., Verrills, N. M., Kavallaris, M., and Gunning, P. W. (2005) Tissue-specific tropomyosin isoform composition, J. Histochem. Cytochem., 53, 557–570.
Schevzov, G., Kee, A. J., Wang, B., Sequeira, V. B., Hook, J., Coombes, J. D., Lucas, C. A., Stehn, J. R., Musgrove, E. A., Cretu, A., Assoian, R., Fath, T., Hanoch, T., Seger, R., Pleines, I., Kile, B. T., Hardeman, E. C., and Gunning, P. W. (2015) Regulation of cell proliferation by ERK and signal-dependent nuclear translocation of ERK is dependent on Tm5NM1-containing actin filaments, Mol. Biol. Cell, 26, 2475–2490.
Pelham, R. J., Jr., Lin, J. J., and Wang, Y. L. (1996) A high molecular mass non-muscle tropomyosin isoform stimulates retrograde organelle transport, J. Cell Sci., 109, 981–989.
Pruyne, D. W., Schott, D. H., and Bretscher, A. (1998) Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast, J. Cell Biol., 143, 1931–1945.
Dalby-Payne, J. R., O’Loughlin, E. V., and Gunning, P. (2003) Polarization of specific tropomyosin isoforms in gastrointestinal epithelial cells and their impact on CFTR at the apical surface, Mol. Biol. Cell, 14, 4365–4375.
Thoms, J. A., Loch, H. M., Bamburg, J. R., Gunning, P. W., and Weinberger, R. P. (2008) A tropomyosin 1 induced defect in cytokinesis can be rescued by elevated expression of cofilin, Cell Motil. Cytoskeleton, 65, 979–990.
McMichael, B. K., and Lee, B. S. (2008) Tropomyosin 4 regulates adhesion structures and resorptive capacity in osteoclasts, Exp. Cell Res., 314, 564–573.
Bach, C. T., Creed, S., Zhong, J., Mahmassani, M., Schevzov, G., Stehn, J., Cowell, L. N., Naumanen, P., Lappalainen, P., Gunning, P. W., and O’Neill, G. M. (2009) Tropomyosin isoform expression regulates the transition of adhesions to determine cell speed and direction, Mol. Cell. Biol., 29, 1506–1514.
O’Neill, G. M. (2009) The coordination between actin filaments and adhesion in mesenchymal migration, Cell Adh. Migr., 3, 355–357.
Caldwell, B. J., Lucas, C., Kee, A. J., Gaus, K., Gunning, P. W., Hardeman, E. C., Yap, A. S., and Gomez, G. A. (2014) Tropomyosin isoforms support actomyosin biogenesis to generate contractile tension at the epithelial zonula adherens, Cytoskeleton, 71, 663–676.
McKillop, D. F., and Geeves, M. A. (1993) Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament, Biophys. J., 65, 693–701.
Al-Khayat, H. A., Yagi, N., and Squire, J. M. (1995) Structural changes in actin–tropomyosin during muscle regulation: computer modelling of low-angle X-ray diffraction data, J. Mol. Biol., 252, 611–632.
Craig, R., and Lehman, W. (2001) Crossbridge and tropomyosin positions observed in native, interacting thick and thin filaments, J. Mol. Biol., 311, 1027–1036.
Bacchiocchi, C., and Lehrer, S. S. (2002) Ca2+-induced movement of tropomyosin in skeletal muscle thin filaments observed by multi-site FRET, Biophys. J., 82, 1524–1536.
Gunning, P. W., Hardeman, E. C., Lappalainen, P., and Mulvihill, D. P. (2015) Tropomyosin–master regulator of actin filament function in the cytoskeleton, J. Cell Sci., 128, 2965–2974.
Khaitlina, S. Y. (2015) Tropomyosin as a regulator of actin dynamics, Int. Rev. Cell Mol. Biol., 318, 255–291.
Manstein, D. J., and Mulvihill, D. P. (2016) Tropomyosin-mediated regulation of cytoplasmic myosins, Traffic, 17, 872–877.
Broschat, K. O. (1990) Tropomyosin prevents depolymerization of actin filaments from the pointed end, J. Biol. Chem., 265, 21323–21329.
Weigt, C., Schoepper, B., and Wegner, A. (1990) Tropomyosin–troponin complex stabilizes the pointed ends of actin filaments against polymerization and depolymerization, FEBS Lett., 260, 266–268.
Kojima, H., Ishijima, A., and Yanagida, T. (1994) Direct measurement of stiffness of single actin filaments with and without tropomyosin by in vitro nanomanipulation, Proc. Natl. Acad. Sci. USA, 91, 12962–12966.
Goldmann, W. H. (2000) Binding of tropomyosin–troponin to actin increases filament bending stiffness, Biochem. Biophys. Res. Commun., 276, 1225–1228.
Watson, M. H., Kuhn, A. E., Novy, R. E., Lin, J. J., and Mak, A. S. (1990) Caldesmon-binding sites on tropomyosin, J. Biol. Chem., 265, 18860–18866.
Maciver, S. K., Ternent, D., and McLaughlin, P. J. (2000) Domain 2 of gelsolin binds directly to tropomyosin, FEBS Lett., 473, 71–75.
Kostyukova, A. S., Choy, A., and Rapp, B. A. (2006) Tropomodulin binds two tropomyosins: a novel model for actin filament capping, Biochemistry, 45, 12068–12075.
Wawro, B., Greenfield, N. J., Wear, M. A., Cooper, J. A., Higgs, H. N., and Hitchcock-DeGregori, S. E. (2007) Tropomyosin regulates elongation by formin at the fast-growing end of the actin filament, Biochemistry, 46, 8146–8155.
Blanchoin, L., Pollard, T. D., and Hitchcock-DeGregori, S. E. (2001) Inhibition of the Arp2/3 complex-nucleated actin polymerization and branch formation by tropomyosin, Curr. Biol., 11, 1300–1304.
Ono, S., and Ono, K. (2002) Tropomyosin inhibits ADF/cofilin-dependent actin filament dynamics, J. Cell Biol., 156, 1065–1076.
Lees-Miller, J. P., and Helfman, D. M. (1991) The molecular basis for tropomyosin isoform diversity, Bioessays, 13, 429–437.
Geeves, M. A., Hitchcock-DeGregori, S. E., and Gunning, P. W. (2015) A systematic nomenclature for mammalian tropomyosin isoforms, J. Muscle Res. Cell Motil., 36, 147–153.
Nevzorov, I. A., and Levitsky, D. I. (2011) Tropomyosin: double helix from the protein world, Biochemistry (Moscow), 76, 1507–1527.
Okumura, N., Hashida-Okumura, A., Kita, K., Matsubae, M., Matsubara, T., Takao, T., and Nagai, K. (2005) Proteomic analysis of slow- and fast-twitch skeletal muscles, Proteomics, 5, 2896–2906.
Oe, M., Ohnishi-Kameyama, M., Nakajima, I., Muroya, S., and Chikuni, K. (2007) Muscle type specific expression of tropomyosin isoforms in bovine skeletal muscles, Meat Sci., 75, 558–563.
Janco, M., Suphamungmee, W., Li, X., Lehman, W., Lehrer, S. S., and Geeves, M. A. (2013) Polymorphism in tropomyosin structure and function, J. Muscle Res. Cell Motil., 34, 177–187.
Muthuchamy, M., Pajak, L., Howles, P., Doetschman, T., and Wieczorek, D. F. (1993) Developmental analysis of tropomyosin gene expression in embryonic stem cells and mouse embryos, Mol. Cell. Biol., 13, 3311–3323.
Muthuchamy, M., Grupp, I. L., Grupp, G., O’Toole, B. A., Kier, A. B., Boivin, G. P., Neumann, J., and Wieczorek, D. F. (1995) Molecular and physiological effects of overex-pressing striated muscle beta-tropomyosin in the adult murine heart, J. Biol. Chem., 270, 30593–30603.
Lehrer, S. S., and Stafford, W. F., 3rd (1991) Preferential assembly of the tropomyosin heterodimer: equilibrium studies, Biochemistry, 30, 5682–5688.
Hvidt, S., and Lehrer, S. S. (1992) Thermally induced chain exchange of frog alpha,beta-tropomyosin, Biophys. Chem., 45, 51–59.
Lehman, W., Vibert, P., Uman, P., and Craig, R. (1995) Steric-blocking by tropomyosin visualized in relaxed vertebrate muscle thin filaments, J. Mol. Biol., 251, 191–196.
Xu, C., Craig, R., Tobacman, L., Horowitz, R., and Lehman, W. (1999) Tropomyosin positions in regulated thin filaments revealed by cryoelectron microscopy, Biophys. J., 77, 985–992.
Behrmann, E., Muller, M., Penczek, P. A., Mannherz, H. G., Manstein, D. J., and Raunser, S. (2012) Structure of the rigor actin–tropomyosin–myosin complex, Cell, 150, 327–338.
Robinson, J. M., Dong, W. J., Xing, J., and Cheung, H. C. (2004) Switching of troponin I: Ca2+ and myosin-induced activation of heart muscle, J. Mol. Biol., 340, 295–305.
Maron, B. J., Gardin, J. M., Flack, J. M., Gidding, S. S., Kurosaki, T. T., and Bild, D. E. (1995) Prevalence of hyper-trophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults, Circulation, 92, 785–789.
Redwood, C., and Robinson, P. (2013) Alpha-tropomyosin mutations in inherited cardiomyopathies, J. Muscle Res. Cell Motil., 34, 285–294.
Watkins, H., Ashrafian, H., and Redwood, C. (2011) Inherited cardiomyopathies, N. Engl. J. Med., 364, 1643–1656.
Bing, W., Knott, A., Redwood, C., Esposito, G., Purcell, I., Watkins, H., and Marston, S. (2000) Effect of hyper-trophic cardiomyopathy mutations in human cardiac muscle alpha-tropomyosin (Asp175Asn and Glu180Gly) on the regulatory properties of human cardiac troponin determined by in vitro motility assay, J. Mol. Cell. Cardiol., 32, 1489–1498.
Michele, D. E., Gomez, C. A., Hong, K. E., Westfall, M. V., and Metzger, J. M. (2002) Cardiac dysfunction in hypertrophic cardiomyopathy mutant tropomyosin mice is transgene-dependent, hypertrophy-independent, and improved by beta-blockade, Circ. Res., 91, 255–262.
Heller, M. J., Nili, M., Homsher, E., and Tobacman, L. S. (2003) Cardiomyopathic tropomyosin mutations that increase thin filament Ca2+ sensitivity and tropomyosin N-domain flexibility, J. Biol. Chem., 278, 41742–41748.
Wang, F., Brunet, N. M., Grubich, J. R., Bienkiewicz, E. A., Asbury, T. M., Compton, L. A., Mihajlovic, G., Miller, V. F., and Chase, P. B. (2011) Facilitated cross-bridge interactions with thin filaments by familial hypertrophic cardiomyopathy mutations in alpha-tropomyosin, J. Biomed. Biotechnol., 2011, 435271.
Geisterfer-Lowrance, A. A., Kass, S., Tanigawa, G., Vosberg, H. P., McKenna, W., Seidman, C. E., and Seidman, J. G. (1990) A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation, Cell, 62, 999–1006.
Richard, P., Charron, P., Carrier, L., Ledeuil, C., Cheav, T., Pichereau, C., Benaiche, A., Isnard, R., Dubourg, O., Burban, M., Gueffet, J. P., Millaire, A., Desnos, M., Schwartz, K., Hainque, B., Komajda, M., and Project, E. H. F. (2003) Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy, Circulation, 107, 2227–2232.
Jaaskelainen, P., Soranta, M., Miettinen, R., Saarinen, L., Pihlajamaki, J., Silvennoinen, K., Tikanoja, T., Laakso, M., and Kuusisto, J. (1998) The cardiac beta-myosin heavy chain gene is not the predominant gene for hypertrophic cardiomyopathy in the Finnish population, J. Am. Coll. Cardiol., 32, 1709–1716.
Tardiff, J. C. (2005) Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes, Heart Failure Rev., 10, 237–248.
Jefferies, J. L., and Towbin, J. A. (2010) Dilated cardiomyopathy, Lancet, 375, 752–762.
Dellefave, L., and McNally, E. M. (2010) The genetics of dilated cardiomyopathy, Curr. Opin. Cardiol., 25, 198–204.
Chang, A. N., Harada, K., Ackerman, M. J., and Potter, J. D. (2005) Functional consequences of hypertrophic and dilated cardiomyopathy-causing mutations in alpha-tropomyosin, J. Biol. Chem., 280, 34343–34349.
Lakdawala, N. K., Dellefave, L., Redwood, C. S., Sparks, E., Cirino, A. L., Depalma, S., Colan, S. D., Funke, B., Zimmerman, R. S., Robinson, P., Watkins, H., Seidman, C. E., Seidman, J. G., McNally, E. M., and Ho, C. Y. (2010) Familial dilated cardiomyopathy caused by an alpha-tropomyosin mutation: the distinctive natural history of sarcomeric dilated cardiomyopathy, J. Am. Coll. Cardiol., 55, 320–329.
Marston, S. B. (2011) How do mutations in contractile proteins cause the primary familial cardiomyopathies? J. Cardiovasc. Transl. Res., 4, 245–255.
Rodriguez Cruz, P. M., Sewry, C., Beeson, D., Jayawant, S., Squier, W., McWilliam, R., and Palace, J. (2014) Congenital myopathies with secondary neuromuscular transmission defects; a case report and review of the literature, Neuromuscul. Disord., 24, 1103–1110.
Tajsharghi, H., Ohlsson, M., Palm, L., and Oldfors, A. (2012) Myopathies associated with beta-tropomyosin mutations, Neuromuscul. Disord., 22, 923–933.
Ochala, J. (2008) Thin filament proteins mutations associated with skeletal myopathies: defective regulation of muscle contraction, J. Mol. Med., 86, 1197–1204.
Marttila, M., Lehtokari, V. L., Marston, S., Nyman, T. A., et al. (2014) Mutation update and genotype–phenotype correlations of novel and previously described mutations in TPM2 and TPM3 causing congenital myopathies, Hum. Mutat., 35, 779–790.
Abicht, A., Dusl, M., Gallenmuller, C., Guergueltcheva, V., Schara, U., Della Marina, A., Wibbeler, E., Almaras, S., Mihaylova, V., von der Hagen, M., Huebner, A., Chaouch, A., Muller, J. S., and Lochmuller, H. (2012) Congenital myasthenic syndromes: achievements and limitations of phenotype-guided gene-after-gene sequencing in diagnostic practice: a study of 680 patients, Hum. Mutat., 33, 1474–1484.
Finlayson, S., Beeson, D., and Palace, J. (2013) Congenital myasthenic syndromes: an update, Pract. Neurol., 13, 80–91.
Orzechowski, M., Fischer, S., Moore, J. R., Lehman, W., and Farman, G. P. (2014) Energy landscapes reveal the myopathic effects of tropomyosin mutations, Arch. Biochem. Biophys., 564, 89–99.
Kremneva, E., Boussouf, S., Nikolaeva, O., Maytum, R., Geeves, M. A., and Levitsky, D. I. (2004) Effects of two familial hypertrophic cardiomyopathy mutations in alpha-tropomyosin, Asp175Asn and Glu180Gly, on the thermal unfolding of actin-bound tropomyosin, Biophys. J., 87, 3922–3933.
Marston, S., Memo, M., Messer, A., Papadaki, M., Nowak, K., McNamara, E., Ong, R., El-Mezgueldi, M., Li, X., and Lehman, W. (2013) Mutations in repeating structural motifs of tropomyosin cause gain of function in skeletal muscle myopathy patients, Hum. Mol. Genet., 22, 4978–4987.
Gupte, T. M., Haque, F., Gangadharan, B., Sunitha, M. S., Mukherjee, S., Anandhan, S., Rani, D. S., Mukundan, N., Jambekar, A., Thangaraj, K., Sowdhamini, R., Sommese, R. F., Nag, S., Spudich, J. A., and Mercer, J. A. (2015) Mechanistic heterogeneity in contractile properties of alpha-tropomyosin (TPM1) mutants associated with inherited cardiomyopathies, J. Biol. Chem., 290, 7003–7015.
Matyushenko, A. M., Shchepkin, D. V., Kopylova, G. V., Popruga, K. E., Artemova, N. V., Pivovarova, A. V., Bershitsky, S. Y., and Levitsky, D. I. (2017) Structural and functional effects of cardiomyopathy-causing mutations in the troponin T-binding region of cardiac tropomyosin, Biochemistry, 56, 250–259.
Farman, G. P., Rynkiewicz, M. J., Orzechowski, M., Lehman, W., and Moore, J. R. (2018) HCM and DCM cardiomyopathy-linked alpha-tropomyosin mutations influence off-state stability and crossbridge interaction on thin filaments, Arch. Biochem. Biophys., 647, 84–92.
Hinkle, A., and Tobacman, L. S. (2003) Folding and function of the troponin tail domain. Effects of cardiomyopath-ic troponin T mutations, J. Biol. Chem., 278, 506–513.
Jagatheesan, G., Rajan, S., Petrashevskaya, N., Schwartz, A., Boivin, G., Arteaga, G., de Tombe, P. P., Solaro, R. J., and Wieczorek, D. F. (2004) Physiological significance of troponin T binding domains in striated muscle tropomyosin, Am. J. Physiol. Heart Circ. Physiol., 287, H1484–1494.
Moore, R. K., Abdullah, S., and Tardiff, J. C. (2014) Allosteric effects of cardiac troponin TNT1 mutations on actomyosin binding: a novel pathogenic mechanism for hypertrophic cardiomyopathy, Arch. Biochem. Biophys., 552–553, 21–28.
Gangadharan, B., Sunitha, M. S., Mukherjee, S., Chowdhury, R. R., Haque, F., Sekar, N., Sowdhamini, R., Spudich, J. A., and Mercer, J. A. (2017) Molecular mechanisms and structural features of cardiomyopathy-causing troponin T mutants in the tropomyosin overlap region, Proc. Natl. Acad. Sci. USA, 114, 11115–11120.
McConnell, M., Tal Grinspan, L., Williams, M. R., Lynn, M. L., Schwartz, B. A., Fass, O. Z., Schwartz, S. D., and Tardiff, J. C. (2017) Clinically divergent mutation effects on the structure and function of the human cardiac tropomyosin overlap, Biochemistry, 56, 3403–3413.
Abdullah, S., Lynn, M. L., McConnell, M. T., Klass, M. M., Baldo, A. P., Schwartz, S. D., and Tardiff, J. C. (2019) FRET-based analysis of the cardiac troponin T linker region reveals the structural basis of the hypertrophic cardiomyopathy-causing Δ160E mutation, J. Biol. Chem., 294, 14634–14647, doi: 10.1074/jbc.RA118.005098.
Heeley, D. H., Golosinska, K., and Smillie, L. B. (1987) The effects of troponin T fragments T1 and T2 on the binding of nonpolymerizable tropomyosin to F-actin in the presence and absence of troponin I and troponin C, J. Biol. Chem., 262, 9971–9978.
Palm, T., Graboski, S., Hitchcock-DeGregori, S. E., and Greenfield, N. J. (2001) Disease-causing mutations in cardiac troponin T: identification of a critical tropomyosin-binding region, Biophys. J., 81, 2827–2837.
Jin, J. P., and Chong, S. M. (2010) Localization of the two tropomyosin-binding sites of troponin T, Arch. Biochem. Biophys., 500, 144–150.
Regitz-Zagrosek, V., Erdmann, J., Wellnhofer, E., Raible, J., and Fleck, E. (2000) Novel mutation in the alpha-tropomyosin gene and transition from hypertrophic to hypocontractile dilated cardiomyopathy, Circulation, 102, E112–116.
Sequeira, V., Wijnker, P. J., Nijenkamp, L. L., Kuster, D. W., Najafi, A., Witjas-Paalberends, E. R., Regan, J. A., Boontje, N., Ten Cate, F. J., Germans, T., Carrier, L., Sadayappan, S., van Slegtenhorst, M. A., Zaremba, R., Foster, D. B., Murphy, A. M., Poggesi, C., Dos Remedios, C., Stienen, G. J., Ho, C. Y., Michels, M., and van der Velden, J. (2013) Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations, Circ. Res., 112, 1491–1505.
Lehrer, S. S., and Geeves, M. A. (2014) The myosin-activated thin filament regulatory state, M(–)-open: a link to hypertrophic cardiomyopathy (HCM), J. Muscle Res. Cell Motil., 35, 153–160.
Lang, R., Gomes, A. V., Zhao, J., Housmans, P. R., Miller, T., and Potter, J. D. (2002) Functional analysis of a troponin I (R145G) mutation associated with familial hypertrophic cardiomyopathy, J. Biol. Chem., 277, 11670–11678.
Kobayashi, T., and Solaro, R. J. (2006) Increased Ca2+ affinity of cardiac thin filaments reconstituted with cardiomyopathy-related mutant cardiac troponin I, J. Biol. Chem., 281, 13471–13477.
Boussouf, S. E., Maytum, R., Jaquet, K., and Geeves, M. A. (2007) Role of tropomyosin isoforms in the calcium sensitivity of striated muscle thin filaments, J. Muscle Res. Cell Motil., 28, 49–58.
Ly, S., and Lehrer, S. S. (2012) Long-range effects of familial hypertrophic cardiomyopathy mutations E180G and D175N on the properties of tropomyosin, Biochemistry, 51, 6413–6420.
Deranek, A. E., Klass, M. M., and Tardiff, J. C. (2019) Moving beyond simple answers to complex disorders in sarcomeric cardiomyopathies: the role of integrated systems, Pflugers’ Arch., 471, 661–671.
Greenfield, N. J., and Fowler, V. M. (2002) Tropomyosin requires an intact N-terminal coiled coil to interact with tropomodulin, Biophys. J., 82, 2580–2591.
Colpan, M., Moroz, N. A., Gray, K. T., Cooper, D. A., Diaz, C. A., and Kostyukova, A. S. (2016) Tropomyosin-binding properties modulate competition between tropomodulin isoforms, Arch. Biochem. Biophys., 600, 23–32.
Colpan, M., Ly, T., Grover, S., Tolkatchev, D., and Kostyukova, A. S. (2017) The cardiomyopathy-associated K15N mutation in tropomyosin alters actin filament pointed end dynamics, Arch. Biochem. Biophys., 630, 18–26.
Ly, T., Pappas, C. T., Johnson, D., Schlecht, W., Colpan, M., Galkin, V. E., Gregorio, C. C., Dong, W. J., and Kostyukova, A. S. (2019) Effects of cardiomyopathy-linked mutations K15N and R21H in tropomyosin on thin-filament regulation and pointed-end dynamics, Mol. Biol. Cell, 30, 268–281.
Moraczewska, J. (2019) Thin filament dysfunctions caused by mutations in tropomyosin Tpm3.12 and Tpm1.1, J. Muscle Res. Cell Motil., July 3, [Epub ahead of print], doi: 10.1007/s10974-019-09532-y.
Clarke, N. F., Waddell, L. B., Sie, L. T., van Bon, B. W., McLean, C., Clark, D., Kornberg, A., Lammens, M., and North, K. N. (2012) Mutations in TPM2 and congenital fiber type disproportion, Neuromusc. Disord., 22, 955–958.
Marttila, M., Lemola, E., Wallefeld, W., Memo, M., Donner, K., Laing, N. G., Marston, S., Gronholm, M., and Wallgren-Pettersson, C. (2012) Abnormal actin binding of aberrant beta-tropomyosins is a molecular cause of muscle weakness in TPM2-related nemaline and cap myopathy, Biochem. J., 442, 231–239.
Robaszkiewicz, K., Dudek, E., Kasprzak, A. A., and Moraczewska, J. (2012) Functional effects of congenital myopathy-related mutations in gamma-tropomyosin gene, Biochim. Biophys. Acta, 1822, 1562–1569.
Memo, M., and Marston, S. (2013) Skeletal muscle myopathy mutations at the actin tropomyosin interface that cause gain- or loss-of-function, J. Muscle Res. Cell Motil., 34, 165–169.
Karpicheva, O. E., Simonyan, A. O., Kuleva, N. V., Redwood, C. S., and Borovikov, Y. S. (2016) Myopathy-causing Q147P TPM2 mutation shifts tropomyosin strands further towards the open position and increases the proportion of strong-binding cross-bridges during the ATPase cycle, Biochim. Biophys. Acta, 1864, 260–267.
Borovikov, Y. S., Rysev, N. A., Karpicheva, O. E., Sirenko, V. V., Avrova, S. V., Piers, A., and Redwood, C. S. (2017) Molecular mechanisms of dysfunction of muscle fibers associated with Glu139 deletion in TPM2 gene, Sci. Rep., 7, 16797.
Borovikov, Y. S., Karpicheva, O. E., Simonyan, A. O., Avrova, S. V., Rogozovets, E. A., Sirenko, V. V., and Redwood, C. S. (2018) The primary causes of muscle dysfunction associated with the point mutations in Tpm3.12; conformational analysis of mutant proteins as a tool for classification of myopathies, Int. J. Mol. Sci., 19, 3975, doi: 10.3390/ijms19123975.
Avrova, S. V., Karpicheva, O. E., Simonyan, A. O., Sirenko, V. V., Redwood, C. S., and Borovikov, Y. S. (2019), The molecular mechanisms of a high Ca2+-sensitivity and muscle weakness associated with the Ala155Thr substitution in Tpm3.12, Biochem. Biophys. Res. Commun., 515, 372–377.
Kostyukova, A. S. (2007) Leiomodin/tropomyosin interactions are isoform specific, Arch. Biochem. Biophys., 465, 227–230.
Kostyukova, A. S., Hitchcock-Degregori, S. E., and Greenfield, N. J. (2007) Molecular basis of tropomyosin binding to tropomodulin, an actin-capping protein, J. Mol. Biol., 372, 608–618.
Ilkovski, B., Mokbel, N., Lewis, R. A., Walker, K., Nowak, K. J., Domazetovska, A., Laing, N. G., Fowler, V. M., North, K. N., and Cooper, S. T. (2008) Disease severity and thin filament regulation in M9R TPM3 nemaline myopathy, J. Neuropathol. Exp. Neurol., 67, 867–877.
Moraczewska, J., Robaszkiewicz, K., Sliwinska, M., Czajkowska, M., Ly, T., Kostyukova, A., Wen, H., and Zheng, W. (2019) Congenital myopathy-related mutations in tropomyosin disrupt regulatory function through altered actin affinity and tropomodulin binding, FEBS J., 286, 1877–1893.
Ochala, J., Lehtokari, V. L., Iwamoto, H., Li, M., Feng, H. Z., Jin, J. P., Yagi, N., Wallgren-Pettersson, C., Penisson-Besnier, I., and Larsson, L. (2011) Disrupted myosin cross-bridge cycling kinetics triggers muscle weakness in nebulin-related myopathy, FASEB J., 25, 1903–1913.
Kiss, B., Lee, E. J., Ma, W., Li, F. W., Tonino, P., Mijailovich, S. M., Irving, T. C., and Granzier, H. L. (2018) Nebulin stiffens the thin filament and augments cross-bridge interaction in skeletal muscle, Proc. Natl. Acad. Sci. USA, 115, 10369–10374.
Marttila, M., Hanif, M., Lemola, E., Nowak, K. J., Laitila, J., Gronholm, M., Wallgren-Pettersson, C., and Pelin, K. (2014) Nebulin interactions with actin and tropomyosin are altered by disease-causing mutations, Skeletal Muscle, 4, 15, doi: 10.1186/2044-5040-4-15.
Loong, C. K., Badr, M. A., and Chase, P. B. (2012) Tropomyosin flexural rigidity and single Ca2+ regulatory unit dynamics: implications for cooperative regulation of cardiac muscle contraction and cardiomyocyte hypertrophy, Front. Physiol., 3, 80, doi: 10.3389/fphys.2012. 00080.
Prabhakar, R., Boivin, G. P., Grupp, I. L., Hoit, B., Arteaga, G., Solaro, R. J., and Wieczorek, D. F. (2001) A familial hypertrophic cardiomyopathy alpha-tropomyosin mutation causes severe cardiac hypertrophy and death in mice, J. Mol. Cell. Cardiol., 33, 1815–1828.
Burkart, E. M., Arteaga, G. M., Sumandea, M. P., Prabhakar, R., Wieczorek, D. F., and Solaro, R. J. (2003) Altered signaling surrounding the C-lobe of cardiac troponin C in myofilaments containing an alpha-tropomyosin mutation linked to familial hypertrophic cardiomyopathy, J. Mol. Cell. Cardiol., 35, 1285–1293.
Nevzorov, I., Redwood, C., and Levitsky, D. (2008) Stability of two beta-tropomyosin isoforms: effects of mutation Arg91Gly, J. Muscle Res. Cell Motil., 29, 173–176.
Borovikov, Y. S., Avrova, S. V., Rysev, N. A., Sirenko, V. V., Simonyan, A. O., Chernev, A. A., Karpicheva, O. E., Piers, A., and Redwood, C. S. (2015) Aberrant movement of beta-tropomyosin associated with congenital myopathy causes defective response of myosin heads and actin during the ATPase cycle, Arch. Biochem. Biophys., 577–578, 11–23.
Akkari, P. A., Song, Y., Hitchcock-DeGregori, S., Blechynden, L., and Laing, N. (2002) Expression and biological activity of baculovirus generated wild-type human slow alpha tropomyosin and the Met9Arg mutant responsible for a dominant form of nemaline myopathy, Biochem. Biophys. Res. Commun., 296, 300–304.
Kalyva, A., Schmidtmann, A., and Geeves, M. A. (2012) In vitro formation and characterization of the skeletal muscle alpha, beta-tropomyosin heterodimers, Biochemistry, 51, 6388–6399.
Perry, S. V. (2001) Vertebrate tropomyosin: distribution, properties and function, J. Muscle Res. Cell Motil., 22, 5–49.
Matyushenko, A. M., Kleymenov, S. Y., Susorov, D. S., and Levitsky, D. I. (2018) Thermal unfolding of homodimers and heterodimers of different skeletal-muscle isoforms of tropomyosin, Biophys. Chem., 243, 1–7.
Lehrer, S. S., and Joseph, D. (1987) Differences in local conformation around cysteine residues in alpha alpha, alpha beta, and beta beta rabbit skeletal tropomyosin, Arch. Biochem. Biophys., 256, 1–9.
Bronson, D. D., and Schachat, F. H. (1982) Heterogeneity of contractile proteins. Differences in tropomyosin in fast, mixed, and slow skeletal muscles of the rabbit, J. Biol. Chem., 257, 3937–3944.
Bicer, S., and Reiser, P. J. (2013) Complex tropomyosin and troponin T isoform expression patterns in orbital and global fibers of adult dog and rat extraocular muscles, J. Muscle Res. Cell Motil., 34, 211–231.
Lehrer, S. S. (1975) Intramolecular crosslinking of tropomyosin via disulfide bond formation: evidence for chain register, Proc. Natl. Acad. Sci. USA, 72, 3377–3381.
Bershitsky, S. Y., Logvinova, D. S., Shchepkin, D. V., Kopylova, G. V., and Matyushenko, A. M. (2019) Myopathic mutations in the beta-chain of tropomyosin differently affect the structural and functional properties of ββ- and αβ-dimers, FASEB J., 33, 1963–1971.
Matyushenko, A. M., Shchepkin, D. V., Susorov, D. S., Nefedova, V. V., Kopylova, G. V., Berg, V. Y., Kleymenov, S. Y., and Levitsky, D. I. (2019) Structural and functional properties of αβ-heterodimers of tropomyosin with myo-pathic mutations Q147P and K49del in the β-chain, Biochem. Biophys. Res. Commun., 508, 934–939.
Janco, M., Kalyva, A., Scellini, B., Piroddi, N., Tesi, C., Poggesi, C., and Geeves, M. A. (2012) α-Tropomyosin with a D175N or E180G mutation in only one chain differs from tropomyosin with mutations in both chains, Biochemistry, 51, 9880–9890.
Funding
Funding. This work was supported by the Russian Foundation for Basic Research (project 17-00-00065).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance with ethical norms. This article does not contain description of studies with human participants or animals performed by any of the authors.
Additional information
Conflict of interest. The authors declare no conflict of interest in financial or any other area.
Russian Text © The Author(s), 2020, published in Uspekhi Biologicheskoi Khimii, 2020, Vol. 60, pp. 43–74.
Rights and permissions
About this article
Cite this article
Matyushenko, A.M., Levitsky, D.I. Molecular Mechanisms of Pathologies of Skeletal and Cardiac Muscles Caused by Point Mutations in the Tropomyosin Genes. Biochemistry Moscow 85 (Suppl 1), 20–33 (2020). https://doi.org/10.1134/S0006297920140023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920140023