Abstract
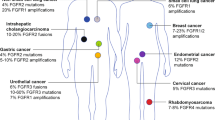
Fibroblast growth factor (FGF) plays an important role in human embryogenesis, angiogenesis, cell proliferation, and differentiation. Carcinogenesis is accompanied by aberrant constitutive activation of FGF receptors (FGFRs) resulting from missense mutation in the FGFR1-4 genes, generation of chimeric oncogenes, FGFR1-4 gene amplification, alternative splicing shift toward formation of mesenchymal FGFR isoforms, and FGFR overexpression. Altogether, these alterations contribute to auto-and paracrine stimulation of cancer cells and neoangiogenesis. Certain missense mutations are found at a high rate in urinary bladder cancer and can be used for non-invasive cancer recurrence diagnostics by analyzing urine cell pellet DNA. Chimeric FGFR1/3 and amplified FGFR1/2 genes can predict cell response to the targeted therapy in various oncological diseases. In recent years, high-throughput sequencing has been used to analyze exomes of virtually all human tumors, which allowed to construct phylogenetic trees of clonal cancer evolution with special emphasis on driver mutations in FGFR1-4 genes. At present, FGFR blockers, such as multi-kinase inhibitors, specific FGFR inhibitors, and FGF ligand traps are being tested in clinical trials. In this review, we discuss current data on the functioning of the FGFR family proteins in both normal and cancer cells, mutations in the FGFR1-4 genes, and mechanisms underlying their oncogenic potential, which might be interesting to a broad range of scientists searching for specific tumor markers and targeted anti-cancer drugs.
Similar content being viewed by others
Abbreviations
- FGF:
-
fibroblast growth factor
- FGFR:
-
FGF receptor
References
Krasil’nikov, M. A., and Zborovskaya, I. B. (2016) Molecular Carcinogenesis [in Russian], ID ABV–press, Moscow.
Helsten, T., Schwaederle, M., and Kurzrock, R. (2015) Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: biologic and clinical implications, Cancer Metastasis Rev., 34, 479–496.
Kushlinskiy, N. E., Mazurenko, N. N., and Nemtsova, M. V. (2016) Molecular and Genetic Tumor Markers [in Russian], RAMN Publisher, Moscow.
Zhang, X., and Zhang, Y. (2015) Bladder cancer and genetic mutations, Cell Biochem. Biophys., 73, 65–69.
Brewer, J. R., Mazot, P., and Soriano, P. (2016) Genetic insights into the mechanisms of Fgf signaling, Genes Dev., 30, 751–771.
Martino, E., Tomlinson, D. C., Williams, S. V., and Knowles, M. A. (2016) A place for precision medicine in bladder cancer: targeting the FGFRs, Future Oncol., 12, 2243–2263.
Touat, M., Ileana, E., Postel–Vinay, S., Andre, F., and Soria, J. C. (2015) Targeting FGFR signaling in cancer, Clin. Cancer Res., 21, 2684–2694.
Salazar, L., Kashiwada, T., Krejci, P., Meyer, A. N., Casale, M., Hallowell, M., Wilcox, W. R., Donoghue, D. J., and Thompson, L. M. (2014) Fibroblast growth factor receptor 3 interacts with and activates TGFbeta–activated kinase 1 tyrosine phosphorylation and NFkappaB signaling in multiple myeloma and bladder cancer, PLoS One, 9, e86470.
De Luca, A., Frezzetti, D., Gallo, M., and Normanno, N. (2017) FGFR–targeted therapeutics for the treatment of breast cancer, Expert Opin. Investig. Drugs, 26, 303–311.
Masoumi–Moghaddam, S., Amini, A., and Morris, D. L. (2014) The developing story of sprouty and cancer, Cancer Metastasis Rev., 33, 695–720.
Winterhoff, B., and Konecny, G. E. (2017) Targeting fibroblast growth factor pathways in endometrial cancer, Curr. Probl. Cancer, 41, 37–47.
Porta, R., Borea, R., Coelho, A., Khan, S., Araujo, A., Reclusa, P., Franchina, T., van der Steen, N., van Dam, P., Ferri, J., Sirera, R., Naing, A., Hong, D., and Rolfo, C. (2017) FGFR a promising druggable target in cancer: molecular biology and new drugs, Crit. Rev. Oncol. Hematol., 113, 256–267.
Zhou, W. Y., Zheng, H., Du, X. L., and Yang, J. L. (2016) Characterization of FGFR signaling pathway as therapeutic targets for sarcoma patients, Cancer Biol. Med., 13, 260–268.
Kaprin, A. D., Starinskiy, V. V., and Petrova, G. V. (2018) 2016 Malignancy Prevalence in Russia (Morbidity and Mortality) [in Russian], Moscow P. A. Gertsen Research Institute of Oncology, Branch of the National Medical Research Radiology Center, Ministry of Health of Russian Federation, Moscow.
Siegel, R. L., Miller, K. D., and Jemal, A. (2018) Cancer statistics, CA Cancer J. Clin., 68, 7–30.
Svatek, R. S., Hollenbeck, B. K., Holmang, S., Lee, R., Kim, S. P., Stenzl, A., and Lotan, Y. (2014) The economics of bladder cancer: costs and considerations of caring for this disease, Eur. Urol., 66, 253–262.
Knowles, M. A., and Hurst, C. D. (2015) Molecular biolo–gy of bladder cancer: new insights into pathogenesis and clinical diversity, Nat. Rev. Cancer, 15, 25–41.
Mikhaylenko, D. S., and Nemtsova, M. V. (2016) Point somatic mutations in developing stomach cancer: key carcinogenesis events, diagnostic markers and therapeutic targets, Urologiya, 1, 100–105.
Knowles, M. A., and Hurst, C. D. (2015) Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity, Nat. Rev. Cancer, 15, 25–41.
Del Piccolo, N., Placone, J., and Hristova, K. (2015) Effect of thanatophoric dysplasia type I mutations on FGFR3 dimerization, Biophys. J., 108, 272–278.
Di Martino, E., Kelly, G., Roulson, J. A., and Knowles, M. A. (2015) Alterations of cell–cell and cell–matrix adhesion in urothelial cells: an oncogenic mechanism for mutant FGFR3, Mol. Cancer Res., 13, 138–148.
Rivera, B., Gayden, T., Carrot–Zhang, J., Nadaf, J., Boshari, T., Faury, D., Zeinieh, M., Blanc, R., Burk, D. L., Fahiminiya, S., Bareke, E., Schuller, U., Monoranu, C. M., Strater, R., Kerl, K., Niederstadt, T., Kurlemann, G., Ellezam, B., Michalak, Z., Thom, M., Lockhart, P. J., Leventer, R. J., Ohm, M., MacGregor, D., Jones, D., Karamchandani, J., Greenwood, C. M., Berghuis, A. M., Bens, S., Siebert, R., Zakrzewska, M., Liberski, P. P., Zakrzewski, K., Sisodiya, S. M., Paulus, W., Albrecht, S., Hasselblatt, M., Jabado, N., Foulkes, W. D., and Majewski, J. (2016) Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors, Acta Neuropathol., 131, 847–863.
Gallo, L. H., Nelson, K. N., Meyer, A. N., and Donoghue, D. J. (2015) Functions of fibroblast growth factor receptors in cancer defined by novel translocations and mutations, Cytokine Growth Factor Rev., 26, 425–449.
Hallinan, N., Finn, S., Cuffe, S., Rafee, S., Byrne, K., and Gately, K. (2016) Targeting the fibroblast growth factor receptor family in cancer, Cancer Treat. Rev., 46, 51–62.
Babina, I. S., and Turner, N. C. (2017) Advances and challenges in targeting FGFR signaling in cancer, Nat. Rev. Cancer, 17, 318–332.
Tanner, Y., and Grose, R. P. (2016) Dysregulating FGF signalling in neoplastic disorders, Semin. Cell Dev. Biol., 53, 126–135.
Weinstein, J. N., Akbani, R., Broom, B. M., Wang, W., Verhaak, R. G., McConkey, D., Lerner, S., Morgan, M., Creighton, C. J., Smith, C., Cherniack, A. D., Kim, J., Sekhar, P. C., Noble, M. S., Al–Ahmadie, H. A., Reuter, V. E., Rosenberg, J. E., Bajorin, D. F., Bochner, B. H., Solit, D. B., et al. (2014) Comprehensive molecular characterization of urothelial bladder carcinoma, Nature, 507, 315–322.
Guo, G., Sun, X., Chen, C., Wu, S., Huang, P., Li, Z., Dean, M., Huang, Y., Jia, W., Zhou, Q., Tang, A., Yang, Z., Li, X., Song, P., Zhao, X., Ye, R., Zhang, S., Lin, Z., Qi, M., Wan, S., Xie, L., Fan, F., Nickerson, M. L., Zou, X., Hu, X., Xing, L., Lv, Z., Mei, H., Gao, S., Liang, C., Gao, Z., Lu, J., Yu, Y., Liu, C., Li, L., Fang, X., Jiang, Z., Yang, J., Li, C., Zhao, X., Chen, J., Zhang, F., Lai, Y., Lin, Z., Zhou, F., Chen, H., Chan, H. C., Tsang, S., Theodorescu, D., Li, Y., Zhang, X., Wang, J., Yang, H., Gui, Y., Wang, J., and Cai, Z. (2013) Whole–genome and whole–exome sequencing of bladder cancer identifies fre–quent alterations in genes involved in sister chromatid cohesion and segregation, Nat. Genet., 45, 1459–1463.
Nakanishi, Y., Akiyama, N., Tsukaguchi, T., Fujii, T., Satoh, Y., Ishii, N., and Aoki, M. (2015) Mechanism of oncogenic signal activation by the novel fusion kinase FGFR3–BAIAP2L1, Mol. Cancer Ther., 14, 704–712.
Katoh, M. (2016) FGFR inhibitors: effects on cancer cells, tumor microenvironment and whole–body homeostasis (review), Int. J. Mol. Med., 38, 3–15.
Arai, Y., Totoki, Y., Hosoda, F., Shirota, T., Hama, N., Nakamura, H., Ojima, H., Furuta, K., Shimada, K., Okusaka, T., Kosuge, T., and Shibata, T. (2014) Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma, Hepatology, 5, 1427–1434.
Fischbach, A., Rogler, A., Erber, R., Stoehr, R., Poulsom, R., Heidenreich, A., Schneevoigt, B. S., Hauke, S., Hartmann, A., Knuechel, R., Veeck, J., and Gaisa, N. T. (2015) Fibroblast growth factor receptor (FGFR) gene amplifications are rare events in bladder cancer, Histopathology, 66, 639–649.
Ross, J. S., Wang, K., Al–Rohil, R. N., Nazeer, T., Sheehan, C. E., Otto, G. A., He, J., Palmer, G., Yelensky, R., Lipson, D., Ali, S., Balasubramanian, S., Curran, J. A., Garcia, L., Mahoney, K., Downing, S. R., Hawryluk, M., Miller, V. A., and Stephens, P. J. (2013) Advanced urothelial carcinoma: next generation sequencing reveals diverse genomic alterations and targets of therapy, Mod. Pathol., 27, 271–280.
Cheng, T., Roth, B., Choi, W., Black, P. C., Dinney, C., and McConkey, D. J. (2013) Fibroblast growth factor receptors–1 and–3 play distinct roles in the regulation of bladder cancer growth and metastasis: implications for therapeutic targeting, PLoS One, 8, e57284.
Andre, F., Bachelot, T., Campone, M., Dalenc, F., Perez–Garcia, J. M., Hurvitz, S. A., Turner, N., Rugo, H., Smith, J. W., Deudon, S., Shi, M., Zhang, Y., Kay, A., Porta, D. G., Yovine, A., and Baselga, J. (2013) Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer, Clin. Cancer Res., 19, 3693–3702.
Kim, S., Dubrovska, A., Salamone, R. J., Walker, J. R., Grandinetti, K. B., Bonamy, G. M., Orth, A. P., Elliott, J., Porta, D. G., Garcia–Echeverria, C., and Reddy, V. A. (2013) FGFR2 promotes breast tumorigenicity through maintenance of breast tumor–initiating cells, PLoS One, 8, e51671.
Andre, F., and Cortes, J. (2015) Rationale for targeting fibroblast growth factor receptor signaling in breast cancer, Breast Cancer Res. Treat., 150, 1–8.
Neuzillet, Y., Van Rhijn, B. W., Prigoda, N. L., Bapat, B., Liu, L., Bostrom, P. J., Fleshner, N. E., Gallie, B. L., Zlotta, A. R., Jewett, M. A., and van der Kwast, T. H. (2014) FGFR3 mutations, but not FGFR3 expression and FGFR3 copy–number variations, are associated with favorable non–muscle invasive bladder cancer, Virchows Arch., 465, 207–213.
Guancial, E. A., Werner, L., Bellmunt, J., Bamias, A., Choueiri, T. K., Ross, R., Schutz, F. A., Park, R. S., Brien, R. J., Hirsch, M. S., Barletta, J. A., Berman, D. M., Lis, R., Loda, M., Stack, E. C., Garraway, L. A., Riester, M., Michor, F., Kantoff, P. W., and Rosenberg, J. E. (2014) FGFR3 expression in primary and metastatic urothelial carcinoma of the bladder, Cancer Med., 3, 835–844.
Parker, B. C., Annala, M. J., Cogdell, D. E., Granberg, K. J., Sun, Y., Ji, P., Li, X., Gumin, J., Zheng, H., Hu, L., Yli–Harja, O., Haapasalo, H., Visakorpi, T., Liu, X., Liu, C. G., Sawaya, R., Fuller, G. N., Chen, K., Lang, F. F., Nykter, M., and Zhang, W. (2013) The tumorigenic FGFR3–TACC3 gene fusion escapes miR–99a regulation in glioblastoma, J. Clin. Invest., 123, 855–865.
Blick, C., Ramachandran, A., Wigfield, S., McCormick, R., Jubb, A., Buffa, F. M., Turley, H., Knowles, M. A., Cranston, D., Catto, J., and Harris, A. L. (2013) Hypoxia regulates FGFR3 expression via HIF–1alpha and miR–100 and contributes to cell survival in non–muscle invasive bladder cancer, Br. J. Cancer, 109, 50–59.
Sfakianos, J. P., Cha, E. K., Iyer, G., Scott, S. N., Zabor, E. C., Shah, R. H., Ren, Q., Bagrodia, A., Kim, P. H., Hakimi, A. A., Ostrovnaya, I., Ramirez, R., Hanrahan, A. J., Desai, N. B., Sun, A., Pinciroli, P., Rosenberg, J. E., Dalbagni, G., Schultz, N., Bajorin, D. F., Reuter, V. E., Berger, M. F., Bochner, B. H., Al–Ahmadie, H. A., Solit, D. B., and Coleman, J. A. (2015) Genomic characterization of upper tract urothelial carcinoma, Eur. Urol., 68, 970–977.
Spiegelberg, C., Giedl, J., Gaisa, N. T., Rogler, A., Riener, M. O., Filbeck, T., Burger, M., Ruemmele, P., Hartmann, A., and Stoehr, R. (2014) Frequency of activating muta–tions in FGFR2 exon 7 in bladder tumors from patients with early–onset and regular–onset disease, Int. J. Clin. Exp. Pathol., 7, 1708–1713.
Bertz, S., Abee, C., Schwarz–Furlan, S., Alfer, J., Hofstadter, F., Stoehr, R., Hartmann, A., and Gaumann, A. K. (2014) Increased angiogenesis and FGFR protein expression indicate a favorable prognosis in bladder cancer, Virchows Arch., 465, 687–695.
Giacomini, A., Chiodelli, P., Matarazzo, S., Rusnati, M., Presta, M., and Ronca, R. (2016) Blocking the FGF/FGFR system as a “two–compartment” antiangiogenic/antitumor approach in cancer therapy, Pharmacol. Res., 107, 172–185.
Katoh, M. (2016) Therapeutics targeting FGF signaling network in human diseases, Trends Pharmacol. Sci., 37, 1081–1096.
Di Meo, A., Bartlett, J., Cheng, Y., Pasic, M. D., and Yousef, G. M. (2017) Liquid biopsy: a step forward towards precision medicine in urologic malignancies, Mol. Cancer, 16, 80.
Noel, N., Couteau, J., Maillet, G., Gobet, F., Aloisio, F., Minier, C., and Pfister, C. (2015) TP53 and FGFR3 gene mutation assessment in urine: pilot study for bladder cancer diagnosis, Anticancer Res., 35, 4915–4921.
Critelli, R., Fasanelli, F., Oderda, M., Polidoro, S., Assumma, M. B., Viberti, C., Preto, M., Gontero, P., Cucchiarale, G., Lurkin, I., Zwarthoff, E. C., Vineis, P., Sacerdote, C., Matullo, G., and Naccarati, A. (2016) Detection of multiple mutations in urinary exfoliated cells from male bladder cancer patients at diagnosis and during follow–up, Oncotarget, 7, 7435–67448.
Togneri, F. S., Ward, D. G., Foster, J. M., Devall, A. J., Wojtowicz, P., Alyas, S., Vasques, F. R., Oumie, A., James, N. D., Cheng, K. K., Zeegers, M. P., Deshmukh, N., Sullivan, B., Taniere, P., Spink, K. G., McMullan, D. J., Griffiths, M., and Bryan, R. T. (2016) Genomic complexity of urothelial bladder cancer revealed in urinary cfDNA, Eur. J. Hum. Genet., 24, 1167–1174.
Karnes, R. J., Fernandez, C. A., and Shuber, A. P. (2012) A noninvasive multianalyte urine–based diagnostic assay for urothelial cancer of the bladder in the evaluation of hematuria, Mayo Clin. Proc., 87, 835–842.
Van Kessel, K. E., Beukers, W., Lurkin, I., Ziel–van der Made, A., van der Keur, K. A., Boormans, J. L., Dyrskjot, L., Marquez, M., Orntoft, T. F., Real, F. X., Segersten, U., Malats, N., Malmstrom, P. U., van Criekinge, W., and Zwarthoff, E. C. (2017) Validation of a DNA methylation–mutation urine assay to select patients with hematuria for cystoscopy, J. Urol., 197, 590–595.
Descotes, F., Kara, N., Decaussin–Petrucci, M., Piaton, E., Geiguer, F., Rodriguez–Lafrasse, C., Terrier, J. E., Lopez, J., and Ruffion, A. (2017) Non–invasive prediction of recurrence in bladder cancer by detecting somatic TERT promoter mutations in urine, Br. J. Cancer, 117, 583–587.
Pivovarcikova, K., Pitra, T., Vanecek, T., Alaghehbandan, R., Gomolcakova, B., Ondic, O., Peckova, K., Rotterova, P., Hora, M., Dusek, M., Michal, M., and Hes, O. (2016) Comparative study of TERT gene mutation analysis on voided liquid–based urine cytology and paraffin–embedded tumorous tissue, Ann. Diagn. Pathol., 24, 7–10.
Ward, D. G., and Bryan, R. T. (2017) Liquid biopsies for bladder cancer, Transl. Androl. Urol., 6, 331–335.
Ross, J. S., Wang, K., Khaira, D., Ali, S. M., Fisher, H. A., Mian, B., Nazeer, T., Elvin, J. A., Palma, N., Yelensky, R., Lipson, D., Miller, V. A., Stephens, P. J., Subbiah, V., and Pal, S. K. (2016) Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations, Cancer, 122, 702–711.
Hedegaard, J., Lamy, P., Nordentoft, I., Algaba, F., Hoyer, S., Ulhoi, B. P., Vang, S., Reinert, T., Hermann, G. G., Mogensen, K., Thomsen, M. B., Nielsen, M. M., Marquez, M., Segersten, U., Aine, M., Hoglund, M., Birkenkamp–Demtroder, K., Fristrup, N., Borre, M., Hartmann, A., Stohr, R., Wach, S., Keck, B., Seitz, A. K., Nawroth, R., Maurer, T., Tulic, C., Simic, T., Junker, K., Horstmann, M., Harving, N., Petersen, A. C., Calle, M. L., Steyerberg, E. W., Beukers, W., van Kessel, K. E. M., Jensen, J. B., Pedersen, J. S., Malmstrom, P. U., Malats, N., Real, F. X., Zwarthoff, E. C., Orntoft, T. F., and Dyrskjot, L. (2016) Comprehensive transcriptional analysis of early–stage urothelial carcinoma, Cancer Cell, 30, 27–42.
Meeks, J. J., Carniero, B. A., Pai, S. G., Oberlin, D. T., Rademaker, A., Fedorchak, K., Balasubramanian, S., Elvin, J., Beaubier, N., and Giles, F. J. (2016) Genomic characterization of high–risk non–muscle invasive bladder cancer, Oncotarget, 7, 75176–75184.
Scott, S. N., Ostrovnaya, I., Lin, C. M., Bouvier, N., Bochner, B. H., Iyer, G., Solit, D., Berger, M. F., and Lin, O. (2017) Next–generation sequencing of urine specimens: a novel platform for genomic analysis in patients with non–muscle–invasive urothelial carcinoma treated with Bacille Calmette–Guerin, Cancer Cytopathol., 125, 416–426.
Ojha, J., Ayres, J., Secreto, C., Tschumper, R., Rabe, K., van Dyke, D., Slager, S., Shanafelt, T., Fonseca, R., Kay, N. E., and Braggio, E. (2015) Deep sequencing identifies genetic heterogeneity and recurrent convergent evolution in chronic lymphocytic leukemia, Blood, 125, 492–498.
Fisher, R., Horswell, S., Rowan, A., Salm, M. P., de Bruin, E. C., Gulati, S., McGranahan, N., Stares, M., Gerlinger, M., Varela, I., Crockford, A., Favero, F., Quidville, V., Andre, F., Navas, C., Gronroos, E., Nicol, D., Hazell, S., Hrouda, D., O’Brien, T., Matthews, N., Phillimore, B., Begum, S., Rabinowitz, A., Biggs, J., Bates, P. A., McDonald, N. Q., Stamp, G., Spencer–Dene, B., Hsieh, J. J., Xu, J., Pickering, L., Gore, M., Larkin, J., and Swanton, C. (2014) Development of synchronous VHL syndrome tumors reveals contingencies and constraints to tumor evolution, Genome Biol., 15, 433.
Aragon–Ching, J. B., and Trump, D. L. (2016) Systematic therapy in muscle–invasive and metastatic bladder cancer: current trends and future promises, Future Oncol., 12, 2049–2058.
Moss, T. J., Qi, Y., Xi, L., Peng, B., Kim, T. B., Ezzedine, N. E., Mosqueda, M. E., Guo, C. C., Czerniak, B. A., Ittmann, M., Wheeler, D. A., Lerner, S. P., and Matin, S. F. (2017) Comprehensive genomic characterization of upper tract urothelial carcinoma, Eur. Urol., 72, 641–649.
Acar, O., Ozkurt, E., Demir, G., Sarac, H., Alkan, C., Esen, T., Somel, M., and Lack, N. A. (2015) Determining the origin of synchronous multifocal bladder cancer by exome sequencing, BMC Cancer, 15, 871.
Pouessel, D., Neuzillet, Y., Mertens, L. S., van der Heijden, M. S., de Jong, J., Sanders, J., Peters, D., Leroy, K., Manceau, A., Maille, P., Soyeux, P., Moktefi, A., Semprez, F., Vordos, D., de la Taille, A., Hurst, C. D., Tomlinson, D. C., Harnden, P., Bostrom, P. J., Mirtti, T., Horenblas, S., Loriot, Y., Houede, N., Chevreau, C., Beuzeboc, P., Shariat, S. F., Sagalowsky, A. I., Ashfaq, R., Burger, M., Jewett, M. A., Zlotta, A. R., Broeks, A., Bapat, B., Knowles, M. A., Lotan, Y., van der Kwast, T. H., Culine, S., Allory, Y., and van Rhijn, B. W. (2016) Tumor heterogeneity of fibroblast growth factor receptor 3 (FGFR3) mutations in invasive bladder cancer: implications for perioperative anti–FGFR3 treatment, Ann. Oncol., 27, 1311–1316.
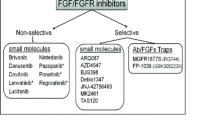
Ho, H. K., Yeo, A. H., Kang, T. S., and Chua, B. T. (2014) Current strategies for inhibiting FGFR activities in clinical applications: opportunities, challenges and toxicological considerations, Drug Discov. Today, 19, 51–62.
Hierro, C., Rodon, J., and Tabernero, J. (2015) Fibroblast growth factor (FGF) receptor/FGF inhibitors: novel targets and strategies for optimization of response of solid tumors, Semin. Oncol., 42, 801–819.
Milowsky, M. I., Dittrich, C., Duran, I., Jagdev, S., Millard, F. E., Sweeney, C. J., Bajorin, D., Cerbone, L., Quinn, D. I., Stadler, W. M., Rosenberg, J. E., Lochheed, M., Sen, P., Squires, M., Shi, M., and Sternberg, C. N. (2014) Phase II trial of dovitinib in patients with progressive FGFR3–mutated or FGFR3 wild–type advanced urothelial carcinoma, Eur. J. Cancer, 50, 3145–3152.
Shah, D. R., Shah, R. R., and Morganroth, J. (2013) Tyrosine kinase inhibitors: their on–target toxicities as potential indicators of efficacy, Drug Saf., 36, 413–426.
Tabernero, J., Bahleda, R., Dienstmann, R., Infante, J. R., Mita, A., Italiano, A., Calvo, E., Moreno, V., Adamo, B., Gazzah, A., Zhong, B., Platero, S. J., Smit, J. W., Stuyckens, K., Chatterjee–Kishore, M., Rodon, J., Peddareddigari, V., Luo, F. R., and Soria, J. C. (2015) Phase I dose–escalation study of JNJ–42756493, an oral pan–fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors, J. Clin. Oncol., 33, 3401–3408.
Jager, W., Xue, H., Hayashi, T., Janssen, C., Awrey, S., Wyatt, A. W., Anderson, S., Moskalev, I., Haegert, A., Alshalalfa, M., Erho, N., Davicioni, E., Fazli, L., Li, E., Collins, C., Wang, Y., and Black, P. C. (2015) Patient–derived bladder cancer xenografts in the preclinical development of novel targeted therapies, Oncotarget, 6, 21522–21532.
Harding, T. C., Long, L., Palencia, S., Zhang, H., Sadra, A., Hestir, K., Patil, N., Levin, A., Hsu, A. W., Charych, D., Brennan, T., Zanghi, J., Halenbeck, R., Marshall, S. A., Qin, M., Doberstein, S. K., Hollenbaugh, D., Kavanaugh, W. M., Williams, L. T., and Baker, K. P. (2013) Blockade of nonhormonal fibroblast growth factor by FP–1039 inhibits growth of multiple types of cancer, Sci. Transl. Med., 5, Suppl. 39.
Presta, M., Chiodelli, P., Giacomini, A., Rusnati, M., and Ronca, R. (2017) Fibroblast growth factors (FGFs) in can–cer: FGF traps as a new therapeutic approach, Pharmacol. Ther., 179, 171–187.
Acquaviva, J., He, S., Zhang, C., Jimenez, J.–P., Nagai, M., Sang, J., Sequeira, M., Smith, D. L., Ogavva, L. S., Takayo, I., Tatsuta, N., Knowles, M. A., Bates, R. C., and Proia, D. A. (2014) FGFR3 translocations in bladder cancer: differential sensitivity to HSP90 inhibition based on drug metabolism, Mol. Cancer Res., 12, 1042–1054.
Carneiro, B. A., Meeks, J. J., Kuzel, T. M., Scaranti, M., Abdulkadir, S. A., and Giles, F. J. (2015) Emerging therapeutic targets in bladder cancer, Cancer Treat. Rev., 41, 170–178.
Yashiro, M., and Matsuoka, T. (2016) Fibroblast growth factor receptor signaling as therapeutic targets in gastric cancer, World J. Gastroenterol., 22, 2415–2423.
Chang, J., Liu, X., Wang, S., Zhang, Z., Wu, Z., Zhang, X., and Li, J. (2014) Prognostic value of FGFR gene amplification in patients with different types of cancer: a systematic review and meta–analysis, PLoS One, 9, e105524.
Herrera–Abreu, M. T., Pearson, A., Campbell, J., Shnyder, S. D., Knowles, M. A., Ashworth, A., and Turner, N. C. (2013) Parallel RNA interference screens identify EGFR activation as an escape mechanism in FGFR3–mutant cancer, Cancer Discov., 3, 1058–1071.
Wang, J., Mikse, O., Liao, R. G., Li, Y., Tan, L., Janne, P. A., Gray, N. S., Wong, K. K., and Hammerman, P. S. (2015) Ligand–associated ERBB2/3 activation confers acquired resistance to FGFR inhibition in FGFR3–dependent cancer cells, Oncogene, 34, 2167–2177.
Chell, V., Balmanno, K., Little, A. S., Wilson, M., Andrews, S., Blockley, L., Hampson, M., Gavine, P. R., and Cook, S. J. (2013) Tumor cell responses to new fibroblast growth factor receptor tyrosine kinase inhibitors and identification of a gatekeeper mutation in FGFR3 as a mechanism of acquired resistance, Oncogene, 32, 3059–3070.
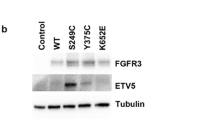
Bunney, T. D., Wan, S., Thiyagarajan, N., Sutto, L., Williams, S. V., Ashford, P., Koss, H., Knowles, M. A., Gervasio, F. L., Coveney, P. V., and Katan, M. (2015) The effect of mutations on drug sensitivity and kinase activity of fibroblast growth factor receptors: a combined experimental and theoretical study, EBioMedicine, 2, 194–204.
Abbosh, P. H., McConkey, D. J., and Plimack, E. R. (2015) Targeting signaling transduction pathways in bladder cancer, Curr. Oncol. Rep., 17, 58.
Van Kessel, K. E., Zuiverloon, T. C., Alberts, A. R., Boormans, J. L., and Zwarthoff, E. C. (2015) Targeted therapies in bladder cancer: an overview of in vivo research, Nat. Rev. Urol., 12, 681–694.
Fedyanin, M. Yu., Khmel’kova, D. N., Serebriyskaya, T. S., Nikol’skaya, T. A., and Tyulyandin, S. A. (2015) Perspectives of therapeutic intervention in FGFR–linked signaling pathway, Uspekhi Mol. Onkol., 1, 27–38.
Chae, Y. K., Ranganath, K., Hammerman, P. S., Vaklavas, C., Mohindra, N., Kalyan, A., Matsangou, M., Costa, R., Carneiro, B., Villaflor, V. M., Cristofanilli, M., and Giles, F. J. (2017) Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application, Oncotarget, 8, 16052–16074.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © D. S. Mikhaylenko, B. Y. Alekseev, D. V. Zaletaev, R. I. Goncharova, M. V. Nemtsova, 2018, published in Biokhimiya, 2018, Vol. 83, No. 8, pp. 1173–1189.
Rights and permissions
About this article
Cite this article
Mikhaylenko, D.S., Alekseev, B.Y., Zaletaev, D.V. et al. Structural Alterations in Human Fibroblast Growth Factor Receptors in Carcinogenesis. Biochemistry Moscow 83, 930–943 (2018). https://doi.org/10.1134/S0006297918080059
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297918080059