Abstract
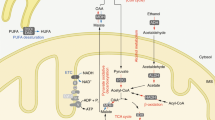
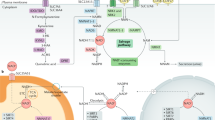
Nicotinamide adenine dinucleotide (NAD) and its phosphorylated form NADP are the major coenzymes in the redox reactions of various essential metabolic pathways. NAD+ also serves as a substrate for several families of regulatory proteins, such as protein deacetylases (sirtuins), ADP-ribosyltransferases, and poly(ADP-ribose) polymerases, that control vital cell processes including gene expression, DNA repair, apoptosis, mitochondrial biogenesis, unfolded protein response, and many others. NAD+ is also a precursor for calcium-mobilizing secondary messengers. Proper regulation of these NAD-dependent metabolic and signaling pathways depends on how efficiently cells can maintain their NAD levels. Generally, mammalian cells regulate their NAD supply through biosynthesis from the precursors delivered with the diet: nicotinamide and nicotinic acid (vitamin B3), as well as nicotinamide riboside and nicotinic acid riboside. Administration of NAD precursors has been demonstrated to restore NAD levels in tissues (i.e., to produce beneficial therapeutic effects) in preclinical models of various diseases, such as neurodegenerative disorders, obesity, diabetes, and metabolic syndrome.
Similar content being viewed by others
Abbreviations
- ADPR:
-
ADP-ribose
- cADPR:
-
cyclic ADP-ribose
- AIF:
-
apoptosis inducing factor
- ARH:
-
ADP-ribosylhydrolase
- ART:
-
ADP-ribosyltransferase
- ARTC:
-
clostridial toxin-like ADP-ribosyltransferase
- ARTD:
-
diphtheria toxin-like ADP-ribosyltransferase
- ER:
-
endoplasmic reticulum
- KDAC:
-
lysine deacetylase
- NA:
-
nicotinic acid
- NAAD:
-
nicotinic acid adenine dinucleotide
- NAADP:
-
nicotinic acid adenine dinucleotide phosphate
- Nam:
-
nicotinamide
- NAMN:
-
nicotinic acid mononucleotide
- NAR:
-
nicotinic acid riboside
- NAPRT:
-
nicotinic acid phosphoribosyltransferase
- NMN:
-
nicotinamide mononucleotide
- NMNAT:
-
nicotinamide mononucleotide adenylyltransferase
- NR:
-
nicotinamide riboside
- PARP:
-
poly(ADP-ribose) polymerase
- Sir2:
-
silent information regulator 2
- SIRT1-7:
-
sirtuins 1-7
References
Houtkooper, R. H., Pirinen, E., and Auwerx, J. (2012) Sirtuins as regulators of metabolism and healthspan, Nat. Rev. Mol. Cell Biol., 13, 225–238.
Hassa, P. O., and Hottiger, M. O. (2008) The diverse bio–logical roles of mammalian PARPS, a small but powerful family of poly–ADP–ribose polymerases, Front. Biosci., 13, 3046–3082.
Nikiforov, A., Kulikova, V., and Ziegler, M. (2015) The human NAD metabolome: functions, metabolism and compartmentalization, Crit. Rev. Biochem. Mol. Biol., 50, 284–297.
Bai, P. (2015) Biology of poly(ADP–ribose) polymerases: the factotums of cell maintenance, Mol. Cell, 58, 947–958.
Feijs, K. L., Verheugd, P., and Luscher, B. (2013) Expanding functions of intracellular resident mono–ADP–ribosylation in cell physiology, FEBS J., 280, 3519–3529.
Fliegert, R., Gasser, A., and Guse, A. H. (2007) Regulation of calcium signalling by adenine–based second messengers, Boichem. Soc. Trans., 35, 109–114.
Belenky, P., Bogan, K. L., and Brenner, C. (2007) NAD+ metabolism in health and disease, Trends Biochem. Sci., 32, 12–19.
Chiarugi, A., Dolle, C., Felici, R., and Ziegler, M. (2012) The NAD metabolome–a key determinant of cancer cell biology, Nat. Rev. Cancer, 12, 741–752.
Katsyuba, E., and Auwerx, J. (2017) Modulating NAD+ metabolism, from bench to bedside, EMBO J., 36, 2670–2683.
Dolle, C., Skoge, R. H., Vanlinden, M. R., and Ziegler, M. (2013) NAD biosynthesis in humans–enzymes, metabo–lites and therapeutic aspects, Curr. Top. Med. Chem., 13, 2907–2917.
Leidecker, O., Bonfiglio, J. J., Colby, T., Zhang, Q., Atanassov, I., Zaja, R., Palazzo, L., Stockum, A., Ahel, I., and Matic, I. (2016) Serine is a new target residue for endogenous ADP–ribosylation on histones, Nat. Chem. Biol., 12, 998–1000.
Daniels, C. M., Ong, S. E., and Leung, A. K. (2014) Phosphoproteomic approach to characterize protein mono–and poly(ADP–ribosyl)ation sites from cells, J. Proteome Res., 13, 3510–3522.
Burkle, A. (2005) Poly(ADP–ribose). The most elaborate metabolite of NAD+, FEBS J., 272, 4576–4589.
Vyas, S., Matic, I., Uchima, L., Rood, J., Zaja, R., Hay, R. T., Ahel, I., and Chang, P. (2014) Family–wide analysis of poly(ADP–ribose) polymerase activity, Nat. Commun., 5, 4426.
Schreiber, V., Ame, J. C., Dolle, P., Schultz, I., Rinaldi, B., Fraulob, V., Menissier–de Murcia, J., and de Murcia, G. (2002) Poly(ADP–ribose) polymerase–2 (PARP–2) is required for efficient base excision DNA repair in association with PARP–1 and XRCC1, J. Biol. Chem., 277, 23028–23036.
De Vos, M., Schreiber, V., and Dantzer, F. (2012) The diverse roles and clinical relevance of PARPs in DNA dam–age repair: current state of the art, Biochem. Pharmacol., 84, 137–146.
Huambachano, O., Herrera, F., Rancourt, A., and Satoh, M. S. (2011) Double–stranded DNA binding domain of poly(ADP–ribose) polymerase–1 and molecular insight into the regulation of its activity, J. Biol. Chem., 286, 7149–7160.
Jungmichel, S., Rosenthal, F., Altmeyer, M., Lukas, J., Hottiger, M. O., and Nielsen, M. L. (2013) Proteome–wide identification of poly(ADP–ribosyl)ation targets in differ–ent genotoxic stress responses, Mol. Cell, 52, 272–285.
Messner, S., Altmeyer, M., Zhao, H., Pozivil, A., Roschitzki, B., Gehrig, P., Rutishauser, D., Huang, D., Caflisch, A., and Hottiger, M. O. (2010) PARP1 ADP–ribosylates lysine residues of the core histone tails, Nucleic Acids Res., 38, 6350–6362.
Poirier, G. G., de Murcia, G., Jongstra–Bilen, J., Niedergang, C., and Mandel, P. (1982) Poly(ADP–ribo–syl)ation of polynucleosomes causes relaxation of chro–matin structure, Proc. Natl. Acad. Sci. USA, 79, 3423–3427.
Krietsch, J., Rouleau, M., Pic, E., Ethier, C., Dawson, T. M., Dawson, V. L., Masson, J. Y., Poirier, G. G., and Gagne, J. P. (2013) Reprogramming cellular events by poly(ADP–ribose)–binding proteins, Mol. Aspects Med., 34, 1066–1087.
Yu, S. W., Wang, H., Poitras, M. F., Coombs, C., Bowers, W. J., Federoff, H. J., Poirier, G. G., Dawson, T. M., and Dawson, V. L. (2002) Mediation of poly(ADP–ribose) polymerase–1–dependent cell death by apoptosis–inducing factor, Science, 297, 259–263.
Wang, H., Shimoji, M., Yu, S. W., Dawson, T. M., and Dawson, V. L. (2003) Apoptosis inducing factor and PARP–mediated injury in the MPTP mouse model of Parkinson’s disease, Ann. N. Y. Acad. Sci., 991, 132–139.
Fouquerel, E., Goellner, E. M., Yu, Z., Gagne, J. P., Barbi de Moura, M., Feinstein, T., Wheeler, D., Redpath, P., Li, J., Romero, G., Migaud, M., Van Houten, B., Poirier, G. G., and Sobol, R. W. (2014) ARTD1/PARP1 negatively regulates glycolysis by inhibiting hexokinase 1 independent of NAD+ depletion, Cell Rep., 8, 1819–1831.
Ha, H. C., and Snyder, S. H. (1999) Poly(ADP–ribose) polymerase is a mediator of necrotic cell death by ATP depletion, Proc. Natl. Acad. Sci. USA, 96, 13978–13982.
Krishnakumar, R., Gamble, M. J., Frizzell, K. M., Berrocal, J. G., Kininis, M., and Kraus, W. L. (2008) Reciprocal binding of PARP–1 and histone H1 at promot–ers specifies transcriptional outcomes, Science, 319, 819–821.
Gibson, B. A., Zhang, Y., Jiang, H., Hussey, K. M., Shrimp, J. H., Lin, H., Schwede, F., Yu, Y., and Kraus, W. L. (2016) Chemical genetic discovery of PARP targets reveals a role for PARP–1 in transcription elongation, Science, 353, 45–50.
Jwa, M., and Chang, P. (2012) PARP16 is a tail–anchored endoplasmic reticulum protein required for the PERK–and IRE1alpha–mediated unfolded protein response, Nat. Cell Biol., 14, 1223–1230.
Feijs, K. L., Kleine, H., Braczynski, A., Forst, A. H., Herzog, N., Verheugd, P., Linzen, U., Kremmer, E., and Luscher, B. (2013) ARTD10 substrate identification on protein microarrays: regulation of GSK3beta by mono–ADP–ribosylation, Cell Commun. Signal., 11, 5.
Mehrotra, P., Riley, J. P., Patel, R., Li, F., Voss, L., and Goenka, S. (2011) PARP–14 functions as a transcriptional switch for Stat6–dependent gene activation, J. Biol. Chem., 286, 1767–1776.
Hottiger, M. O., Hassa, P. O., Luscher, B., Schuler, H., and Koch–Nolte, F. (2010) Toward a unified nomenclature for mammalian ADP–ribosyltransferases, Trends Biochem. Sci., 35, 208–219.
Jiang, H., Khan, S., Wang, Y., Charron, G., He, B., Sebastian, C., Du, J., Kim, R., Ge, E., Mostoslavsky, R., Hang, H. C., Hao, Q., and Lin, H. (2013) SIRT6 regulates TNF–alpha secretion through hydrolysis of long–chain fatty acyl lysine, Nature, 496, 110–113.
Martin, A., Tegla, C. A., Cudrici, C. D., Kruszewski, A. M., Azimzadeh, P., Boodhoo, D., Mekala, A. P., Rus, V., and Rus, H. (2015) Role of SIRT1 in autoimmune demyelination and neurodegeneration, Immunol. Res., 61, 187–197.
Kuny, C. V., and Sullivan, C. S. (2016) Virus–host interac–tions and the ARTD/PARP family of enzymes, PLoS Pathog., 12, e1005453.
Yang, C. S., Jividen, K., Spencer, A., Dworak, N., Ni, L., Oostdyk, L. T., Chatterjee, M., Kusmider, B., Reon, B., Parlak, M., Gorbunova, V., Abbas, T., Jeffery, E., Sherman, N. E., and Paschal, B. M. (2017) Ubiquitin modification by the E3 ligase/ADP–ribosyltransferase Dtx3L/Parp9, Mol. Cell, 66, 503–516 e505.
Seman, M., Adriouch, S., Haag, F., and Koch–Nolte, F. (2004) Ecto–ADP–ribosyltransferases (ARTs): emerging actors in cell communication and signaling, Curr. Med. Chem., 11, 857–872.
Mashimo, M., Kato, J., and Moss, J. (2013) ADP–ribosyl–acceptor hydrolase 3 regulates poly (ADP–ribose) degrada–tion and cell death during oxidative stress, Proc. Natl. Acad. Sci. USA, 110, 18964–18969.
Niere, M., Mashimo, M., Agledal, L., Dolle, C., Kasamatsu, A., Kato, J., Moss, J., and Ziegler, M. (2012) ADP–ribosylhydrolase 3 (ARH3), not poly(ADP–ribose) glycohydrolase (PARG) isoforms, is responsible for degra–dation of mitochondrial matrix–associated poly(ADP–ribose), J. Biol. Chem., 287, 16088–16102.
Slade, D., Dunstan, M. S., Barkauskaite, E., Weston, R., Lafite, P., Dixon, N., Ahel, M., Leys, D., and Ahel, I. (2011) The structure and catalytic mechanism of a poly(ADP–ribose) glycohydrolase, Nature, 477, 616–620.
Jankevicius, G., Hassler, M., Golia, B., Rybin, V., Zacharias, M., Timinszky, G., and Ladurner, A. G. (2013) A family of macrodomain proteins reverses cellular mono–ADP–ribosylation, Nat. Struct. Mol. Biol., 20, 508–514.
Sharifi, R., Morra, R., Appel, C. D., Tallis, M., Chioza, B., Jankevicius, G., Simpson, M. A., Matic, I., Ozkan, E., Golia, B., Schellenberg, M. J., Weston, R., Williams, J. G., Rossi, M. N., Galehdari, H., Krahn, J., Wan, A., Trembath, R. C., Crosby, A. H., Ahel, D., Hay, R., Ladurner, A. G., Timinszky, G., Williams, R. S., and Ahel, I. (2013) Deficiency of terminal ADP–ribose protein glyco–hydrolase TARG1/C6orf130 in neurodegenerative disease, EMBO J., 32, 1225–1237.
Fontana, P., Bonfiglio, J. J., Palazzo, L., Bartlett, E., Matic, I., and Ahel, I. (2017) Serine ADP–ribosylation reversal by the hydrolase ARH3, Elife, 6, e28533.
Moss, J., Stanley, S. J., Nightingale, M. S., Murtagh, J. J., Jr., Monaco, L., Mishima, K., Chen, H. C., Williamson, K. C., and Tsai, S. C. (1992) Molecular and immunological characterization of ADP–ribosylarginine hydrolases, J. Biol. Chem., 267, 10481–10488.
Palazzo, L., Thomas, B., Jemth, A. S., Colby, T., Leidecker, O., Feijs, K. L., Zaja, R., Loseva, O., Puigvert, J. C., Matic, I., Helleday, T., and Ahel, I. (2015) Processing of protein ADP–ribosylation by Nudix hydrolases, Biochem. J., 468, 293–301.
Talhaoui, I., Lebedeva, N. A., Zarkovic, G., Saint–Pierre, C., Kutuzov, M. M., Sukhanova, M. V., Matkarimov, B. T., Gasparutto, D., Saparbaev, M. K., Lavrik, O. I., and Ishchenko, A. A. (2016) Poly(ADP–ribose) polymerases covalently modify strand break termini in DNA fragments in vitro, Nucleic Acids Res., 44, 9279–9295.
Munnur, D., and Ahel, I. (2017) Reversible mono–ADP–ribosylation of DNA breaks, FEBS J., 284, 4002–4016.
Kaelin, W. G., Jr., and McKnight, S. L. (2013) Influence of metabolism on epigenetics and disease, Cell, 153, 56–69.
Roth, S. Y., Denu, J. M., and Allis, C. D. (2001) Histone acetyltransferases, Annu. Rev. Biochem., 70, 81–120.
Haberland, M., Montgomery, R. L., and Olson, E. N. (2009) The many roles of histone deacetylases in develop–ment and physiology: implications for disease and therapy, Nat. Rev. Genet., 10, 32–42.
Brachmann, C. B., Sherman, J. M., Devine, S. E., Cameron, E. E., Pillus, L., and Boeke, J. D. (1995) The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromo–some stability, Genes Dev., 9, 2888–2902.
Tanner, K. G., Landry, J., Sternglanz, R., and Denu, J. M. (2000) Silent information regulator 2 family of NAD–dependent histone/protein deacetylases generates a unique product, 1–O–acetyl–ADP–ribose, Proc. Natl. Acad. Sci. USA, 97, 14178–14182.
Michishita, E., Park, J. Y., Burneskis, J. M., Barrett, J. C., and Horikawa, I. (2005) Evolutionarily conserved and non–conserved cellular localizations and functions of human SIRT proteins, Mol. Biol. Cell, 16, 4623–4635.
Imai, S., Armstrong, C. M., Kaeberlein, M., and Guarente, L. (2000) Transcriptional silencing and longevi–ty protein Sir2 is an NAD–dependent histone deacetylase, Nature, 403, 795–800.
Landry, J., Sutton, A., Tafrov, S. T., Heller, R. C., Stebbins, J., Pillus, L., and Sternglanz, R. (2000) The silencing pro–tein SIR2 and its homologs are NAD–dependent protein deacetylases, Proc. Natl. Acad. Sci. USA, 97, 5807–5811.
Senawong, T., Peterson, V. J., and Leid, M. (2005) BCL11A–dependent recruitment of SIRT1 to a promoter template in mammalian cells results in histone deacetyla–tion and transcriptional repression, Arch. Biochem. Biophys., 434, 316–325.
Vaquero, A., Scher, M., Lee, D., Erdjument–Bromage, H., Tempst, P., and Reinberg, D. (2004) Human SirT1 inter–acts with histone H1 and promotes formation of facultative heterochromatin, Mol. Cell, 16, 93–105.
Martinez–Redondo, P., and Vaquero, A. (2013) The diver–sity of histone versus nonhistone sirtuin substrates, Genes Cancer, 4, 148–163.
Sawada, M., Sun, W., Hayes, P., Leskov, K., Boothman, D. A., and Matsuyama, S. (2003) Ku70 suppresses the apop–totic translocation of Bax to mitochondria, Nat. Cell Biol., 5, 320–329.
Bosch–Presegue, L., and Vaquero, A. (2011) The dual role of sirtuins in cancer, Genes Cancer, 2, 648–662.
Kaidi, A., Weinert, B. T., Choudhary, C., and Jackson, S. P. (2010) Human SIRT6 promotes DNA end resection through CtIP deacetylation, Science, 329, 1348–1353.
Lin, S. J., Ford, E., Haigis, M., Liszt, G., and Guarente, L. (2004) Calorie restriction extends yeast life span by lower–ing the level of NADH, Genes Dev., 18, 12–16.
Desquiret–Dumas, V., Gueguen, N., Leman, G., Baron, S., Nivet–Antoine, V., Chupin, S., Chevrollier, A., Vessieres, E., Ayer, A., Ferre, M., Bonneau, D., Henrion, D., Reynier, P., and Procaccio, V. (2013) Resveratrol induces a mitochondrial complex I–dependent increase in NADH oxidation responsible for sirtuin activation in liver cells, J. Biol. Chem., 288, 36662–36675.
Lagouge, M., Argmann, C., Gerhart–Hines, Z., Meziane, H., Lerin, C., Daussin, F., Messadeq, N., Milne, J., Lambert, P., Elliott, P., Geny, B., Laakso, M., Puigserver, P., and Auwerx, J. (2006) Resveratrol improves mitochon–drial function and protects against metabolic disease by activating SIRT1 and PGC–1alpha, Cell, 127, 1109–1122.
Jiang, W., Wang, S., Xiao, M., Lin, Y., Zhou, L., Lei, Q., Xiong, Y., Guan, K. L., and Zhao, S. (2011) Acetylation reg–ulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase, Mol. Cell, 43, 33–44.
North, B. J., Marshall, B. L., Borra, M. T., Denu, J. M., and Verdin, E. (2003) The human Sir2 ortholog, SIRT2, is an NAD+–dependent tubulin deacetylase, Mol. Cell, 11, 437–444.
Schlicker, C., Gertz, M., Papatheodorou, P., Kachholz, B., Becker, C. F., and Steegborn, C. (2008) Substrates and reg–ulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5, J. Mol. Biol., 382, 790–801.
Mao, Z., Hine, C., Tian, X., Van Meter, M., Au, M., Vaidya, A., Seluanov, A., and Gorbunova, V. (2011) SIRT6 promotes DNA repair under stress by activating PARP1, Science, 332, 1443–1446.
Du, J., Zhou, Y., Su, X., Yu, J. J., Khan, S., Jiang, H., Kim, J., Woo, J., Kim, J. H., Choi, B. H., He, B., Chen, W., Zhang, S., Cerione, R. A., Auwerx, J., Hao, Q., and Lin, H. (2011) Sirt5 is a NAD–dependent protein lysine demalonylase and desuccinylase, Science, 334, 806–809.
Haigis, M. C., Mostoslavsky, R., Haigis, K. M., Fahie, K., Christodoulou, D. C., Murphy, A. J., Valenzuela, D. M., Yancopoulos, G. D., Karow, M., Blander, G., Wolberger, C., Prolla, T. A., Weindruch, R., Alt, F. W., and Guarente, L. (2006) SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells, Cell, 126, 941–954.
Sassone–Corsi, P. (2016) The epigenetic and metabolic lan–guage of the circadian clock, in A Time for Metabolism and Hormones (Sassone–Corsi, P., and Christen, Y., eds.), Springer, pp. 1–11.
Imai, S., and Guarente, L. (2014) NAD+ and sirtuins in aging and disease, Trends Cell. Biol., 24, 464–471.
Cao, Y., Jiang, X., Ma, H., Wang, Y., Xue, P., and Liu, Y. (2016) SIRT1 and insulin resistance, J. Diabetes Complications, 30, 178–183.
Lee, H. C. (2012) Cyclic ADP–ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization, J. Biol. Chem., 287, 31633–31640.
Sumoza–Toledo, A., and Penner, R. (2011) TRPM2: a mul–tifunctional ion channel for calcium signalling, J. Physiol., 589, 1515–1525.
Malavasi, F., Deaglio, S., Funaro, A., Ferrero, E., Horenstein, A. L., Ortolan, E., Vaisitti, T., and Aydin, S. (2008) Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology, Physiol. Rev., 88, 841–886.
Bruzzone, S., Guida, L., Zocchi, E., Franco, L., and De Flora, A. (2001) Connexin 43 hemi channels mediate Ca2+–regulated transmembrane NAD+ fluxes in intact cells, FASEB J., 15, 10–12.
Podesta, M., Benvenuto, F., Pitto, A., Figari, O., Bacigalupo, A., Bruzzone, S., Guida, L., Franco, L., Paleari, L., Bodrato, N., Usai, C., De Flora, A., and Zocchi, E. (2005) Concentrative uptake of cyclic ADP–ribose generated by BST–1+ stroma stimulates proliferation of human hematopoietic progenitors, J. Biol. Chem., 280, 5343–5349.
Franco, L., Guida, L., Bruzzone, S., Zocchi, E., Usai, C., and De Flora, A. (1998) The transmembrane glycoprotein CD38 is a catalytically active transporter responsible for generation and influx of the second messenger cyclic ADP–ribose across membranes, FASEB J., 12, 1507–1520.
Song, E. K., Rah, S. Y., Lee, Y. R., Yoo, C. H., Kim, Y. R., Yeom, J. H., Park, K. H., Kim, J. S., Kim, U. H., and Han, M. K. (2011) Connexin–43 hemichannels mediate cyclic ADP–ribose generation and its Ca2+–mobilizing activity by NAD+/cyclic ADP–ribose transport, J. Biol. Chem., 286, 44480–44490.
Zhao, Y. J., Zhu, W. J., Wang, X. W., Zhang, L. H., and Lee, H. C. (2015) Determinants of the membrane orienta–tion of a calcium signaling enzyme CD38, Biochim. Biophys. Acta, 1853, 2095–2103.
Bieganowski, P., and Brenner, C. (2004) Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss–Handler independent route to NAD+ in fungi and humans, Cell, 117, 495–502.
Kulikova, V., Shabalin, K., Nerinovski, K., Dolle, C., Niere, M., Yakimov, A., Redpath, P., Khodorkovskiy, M., Migaud, M. E., Ziegler, M., and Nikiforov, A. (2015) Generation, release, and uptake of the NAD precursor nicotinic acid riboside by human cells, J. Biol. Chem., 290, 27124–27137.
Guillemin, G. J., and Brew, B. J. (2002) Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s dis–ease, Redox Rep., 7, 199–206.
Bahn, A., Hagos, Y., Reuter, S., Balen, D., Brzica, H., Krick, W., Burckhardt, B. C., Sabolic, I., and Burckhardt, G. (2008) Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13), J. Biol. Chem., 283, 16332–16341.
Gopal, E., Miyauchi, S., Martin, P. M., Ananth, S., Roon, P., Smith, S. B., and Ganapathy, V. (2007) Transport of nicotinate and structurally related compounds by human SMCT1 (SLC5A8) and its relevance to drug transport in the mammalian intestinal tract, Pharm. Res., 24, 575–584.
Kanai, Y., Segawa, H., Miyamoto, K., Uchino, H., Takeda, E., and Endou, H. (1998) Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98), J. Biol. Chem., 273, 23629–23632.
Pillai, S. M., and Meredith, D. (2011) SLC36A4 (hPAT4) is a high affinity amino acid transporter when expressed in Xenopus laevis oocytes, J. Biol. Chem., 286, 2455–2460.
Nikiforov, A., Dolle, C., Niere, M., and Ziegler, M. (2011) Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular pre–cursors to mitochondrial NAD generation, J. Biol. Chem., 286, 21767–21778.
VanLinden, M. R., Dolle, C., Pettersen, I. K., Kulikova, V. A., Niere, M., Agrimi, G., Dyrstad, S. E., Palmieri, F., Nikiforov, A. A., Tronstad, K. J., and Ziegler, M. (2015) Subcellular distribution of NAD+ between cytosol and mitochondria determines the metabolic profile of human cells, J. Biol. Chem., 290, 27644–27659.
Pittelli, M., Formentini, L., Faraco, G., Lapucci, A., Rapizzi, E., Cialdai, F., Romano, G., Moneti, G., Moroni, F., and Chiarugi, A. (2010) Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool, J. Biol. Chem., 285, 34106–34114.
Dolle, C., Niere, M., Lohndal, E., and Ziegler, M. (2010) Visualization of subcellular NAD pools and intra–organel–lar protein localization by poly–ADP–ribose formation, Cell. Mol. Life Sci., 67, 433–443.
Van Linden, M. R., Niere, M., Nikiforov, A. A., Ziegler, M., and Dolle, C. (2017) Compartment–specific poly–ADP–ribose formation as a biosensor for subcellular NAD pools, Methods Mol. Biol., 1608, 45–56.
Agrimi, G., Russo, A., Scarcia, P., and Palmieri, F. (2012) The human gene SLC25A17 encodes a peroxisomal trans–porter of coenzyme A, FAD and NAD+, Biochem. J., 443, 241–247.
Senesi, S., Csala, M., Marcolongo, P., Fulceri, R., Mandl, J., Banhegyi, G., and Benedetti, A. (2010) Hexose–6–phos–phate dehydrogenase in the endoplasmic reticulum, Biol. Chem., 391, 1–8.
Fabrizio, G., Di Paola, S., Stilla, A., Giannotta, M., Ruggiero, C., Menzel, S., Koch–Nolte, F., Sallese, M., and Di Girolamo, M. (2015) ARTC1–mediated ADP–ribosyla–tion of GRP78/BiP: a new player in endoplasmic reticulum stress responses, Cell. Mol. Life Sci., 72, 1209–1225.
Williams, P. A., Harder, J. M., Foxworth, N. E., Cochran, K. E., Philip, V. M., Porciatti, V., Smithies, O., and John, S. W. (2017) Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice, Science, 355, 756–760.
Gong, B., Pan, Y., Vempati, P., Zhao, W., Knable, L., Ho, L., Wang, J., Sastre, M., Ono, K., Sauve, A. A., and Pasinetti, G. M. (2013) Nicotinamide riboside restores cog–nition through an upregulation of proliferator–activated receptor–gamma coactivator 1alpha regulated beta–secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models, Neurobiol. Aging, 34, 1581–1588.
Brown, K. D., Maqsood, S., Huang, J. Y., Pan, Y., Harkcom, W., Li, W., Sauve, A., Verdin, E., and Jaffrey, S. R. (2014) Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise–induced hear–ing loss, Cell Metab., 20, 1059–1068.
Trammell, S. A., Weidemann, B. J., Chadda, A., Yorek, M. S., Holmes, A., Coppey, L. J., Obrosov, A., Kardon, R. H., Yorek, M. A., and Brenner, C. (2016) Nicotinamide ribo–side opposes type 2 diabetes and neuropathy in mice, Sci. Rep., 6, 26933.
Canto, C., Houtkooper, R. H., Pirinen, E., Youn, D. Y., Oosterveer, M. H., Cen, Y., Fernandez–Marcos, P. J., Yamamoto, H., Andreux, P. A., Cettour–Rose, P., Gademann, K., Rinsch, C., Schoonjans, K., Sauve, A. A., and Auwerx, J. (2012) The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high–fat diet–induced obesity, Cell Metab., 15, 838–847.
Khan, N. A., Auranen, M., Paetau, I., Pirinen, E., Euro, L., Forsstrom, S., Pasila, L., Velagapudi, V., Carroll, C. J., Auwerx, J., and Suomalainen, A. (2014) Effective treat–ment of mitochondrial myopathy by nicotinamide ribo–side, a vitamin B3, EMBO Mol. Med., 6, 721–731.
Cerutti, R., Pirinen, E., Lamperti, C., Marchet, S., Sauve, A. A., Li, W., Leoni, V., Schon, E. A., Dantzer, F., Auwerx, J., Viscomi, C., and Zeviani, M. (2014) NAD+–dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease, Cell Metab., 19, 1042–1049.
Yoshino, J., Mills, K. F., Yoon, M. J., and Imai, S. (2011) Nicotinamide mononucleotide, a key NAD+ intermedi–ate, treats the pathophysiology of diet–and age–induced diabetes in mice, Cell Metab., 14, 528–536.
Yamamoto, T., Byun, J., Zhai, P., Ikeda, Y., Oka, S., and Sadoshima, J. (2014) Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion, PLoS One, 9, e98972.
Mills, K. F., Yoshida, S., Stein, L. R., Grozio, A., Kubota, S., Sasaki, Y., Redpath, P., Migaud, M. E., Apte, R. S., Uchida, K., Yoshino, J., and Imai, S. I. (2016) Long–term administration of nicotinamide mononucleotide mitigates age–associated physiological decline in mice, Cell Metab., 24, 795–806.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V. A. Kulikova, D. V. Gromyko, A. A. Nikiforov, 2018, published in Biokhimiya, 2018, Vol. 83, No. 7, pp. 987–1001.
Rights and permissions
About this article
Cite this article
Kulikova, V.A., Gromyko, D.V. & Nikiforov, A.A. The Regulatory Role of NAD in Human and Animal Cells. Biochemistry Moscow 83, 800–812 (2018). https://doi.org/10.1134/S0006297918070040
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297918070040